28 september 2015: Bekijk ook deze lijst mocht u een beenmerg- of stamceltransplantatie moeten ondergaan:
https://kanker-actueel.nl/NL/studiepublicaties-van-niet-toxische-middelen-en-behandelingen-uit-literatuurlijst-van-arts-bioloog-drs-engelbert-valstar-naast-beenmergtransplantaties-en-stamceltransplantaties.html
28 september 2015: Een recente studie geeft een overzicht hoever het gebruik van navelstrwengbloed is gevorderd bij stamceltransplantaties bij vormen van leukemie als er geen goede donor is te vinden.
Onderaan staat het abstract van deze studie: Concise Review: Umbilical Cord Blood Transplantation: Past, Present, and Future waarvan het volledige studierapport gratis is in te zien en met een interessante referentielijst die ik ook onderaan dit artikel heb geplaatst.
28 november 2004: Bron: New England Journal of Medicine
Twee studies waaronder een Europese studie bewijst dat bloed van de navelstreng c.q. placenta volwassenen met leukemie kan genezen als er geen goede beschikbare donor is voor stamceltransplantatie of beenmergtransplantaties. Lees ook verhaal van Nederlandse man die geneest door bloed van zijn eigen kind onder deze artikelen en twee jaar geleden al geplaatst. Morgen hopelijk wat meer vertaald uit onderstaande artikelen en studies in het Nederlands. Eerst een artikel c.q. persbericht en daaronder de abstracten van de twee studies waarover hier gesproken wordt.
Studies: Cord Blood Works Vs. Leukemia
November 24, 2004
Umbilical-cord blood, now used mostly to treat children with leukemia, could save thousands of adults with the disease each year who cannot find bone marrow donors, two big studies indicate.
A European study found that those who got cord blood were just as likely to be free of leukemia two years later as those who got marrow. A U.S. study looking at three-year survival yielded results almost as promising.
To Dr. Mary Horowitz of the Medical College of Wisconsin, senior author of the U.S. study, the message is clear: Umbilical cord blood can save adults.
Leukemia patients often undergo radiation or chemotherapy to kill their cancerous white blood cells _ a treatment that wipes out their immune systems, too. To restore their immune systems, doctors give these patients an infusion of bone marrow or umbilical cord blood, both of which contain stem cells capable of developing into every kind of blood cell.
Cord blood offers an important advantage over marrow that makes it particularly valuable for use in transplants: Its stem cells are less likely to attack the recipient's body. That allows a wider margin of error in matching up donors and recipients.
But up to now, cord blood has been considered suitable only for children, because each donation has only about one-tenth the number of stem cells in a marrow donation.
The two new studies, published in Thursday's New England Journal of Medicine, suggest that is not a serious impediment.
In the European study, involving 682 patients, about one-third of both those who got matched marrow and those who got cord blood that did not quite match their own tissues were alive after two years. In the U.S. study of 601 patients, about one-third of those who got matched marrow were leukemia-free after two years, compared with about one-fifth of those who got cord blood or unmatched marrow.
Both studies were based on records from transplants in the late 1990s and early 2000s.
Using cord blood could improve the odds of getting a transplant for the 16,000 U.S. adult leukemia patients each year who cannot find a compatible marrow donor, said the U.S. study's leader, Dr. Mary J. Laughlin of Case Comprehensive Cancer Center in Cleveland.
Still, Dr. Nancy Kernan, assistant chief of marrow transplantation at Memorial-Sloan-Kettering Cancer Center in New York, said cord blood transplants in adults should be done only as part of studies to look at and improve their effectiveness.
Public cord blood banks _ where blood drawn from umbilical cords and placentas at birth is kept frozen _ need to quadruple their supply to find a match for every leukemia patient who needs one. With 4 million births a year in this country, and most cord blood thrown away, that should not be a problem once more public money comes into play, doctors said.
A federal Institute of Medicine committee is already looking into the best way to set up a national cord blood supply, and is scheduled to complete its report in March.
"I know our committee will consume this study avidly," said Kristine Gebbie, chairman of the group.
The first bone marrow transplants were done in the 1960s; cord blood transplants started in the 1990s. Stem-cell transplants save only 20 percent to 30 percent of the patients who hope to grow new immune systems. But without the treatment, virtually all of them would die.
Some researchers said techniques they have developed in the past two years, since the study ended, already have boosted their success.
Most doctors consider cord blood more appropriate for smaller people, because it contains fewer stem cells than marrow. In the two studies, cord blood recipients tended to weigh less than those who got marrow _ an average of 22 pounds less in the U.S. research, about 18 in the European study.
There are two competing U.S. public cord bank systems, one holding about 38,000 vials, the other 27,000. Together, they do not add up to the supply kept by just one of the 20 or so private banks kept for paying families.
Het eerste studieabstract is deze gehaald uit British Journal of Medicin Outcomes after Transplantation of Cord Blood or Bone Marrow from Unrelated Donors in Adults with Leukemia
Mary J. Laughlin, M.D., Mary Eapen, M.B., B.S., Pablo Rubinstein, M.D., John E. Wagner, M.D., Mei-Jei Zhang, Ph.D., Richard E. Champlin, M.D., Cladd Stevens, M.D., Juliet N. Barker, M.D., Robert P. Gale, M.D., Ph.D., Hillard M. Lazarus, M.D., David I. Marks, M.D., Ph.D., Jon J. van Rood, M.D., Andromachi Scaradavou, M.D., and Mary M. Horowitz, M.D.
ABSTRACT
Background Data regarding the outcome of cord-blood transplantation in adults are scant, despite the fact that these grafts are increasingly used in adults.
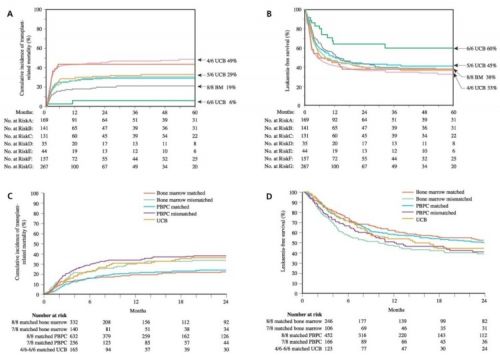
Methods We compared the outcomes of the transplantation of hematopoietic stem cells from unrelated donors in adults with leukemia who had received cord blood that was mismatched for one HLA antigen (34 patients) or two antigens (116 patients), bone marrow that had one HLA mismatch (83 patients), and HLA-matched bone marrow (367 patients). We used Cox proportional-hazards models to analyze the data.
Results Cord-blood recipients were younger and more likely to have advanced leukemia than were bone marrow recipients, and they received lower doses of nucleated cells. Hematopoietic recovery was slower with transplantation of mismatched bone marrow and cord blood than with matched marrow transplantations. Acute graft-versus-host disease (GVHD) was more likely to occur after mismatched marrow transplantation, and chronic GVHD was more likely to occur after cord-blood transplantation. The rates of treatment-related mortality, treatment failure, and overall mortality were lowest among patients who received matched marrow transplants. Patients who received mismatched bone marrow transplants and those who received mismatched cord-blood transplants had similar rates of treatment-related mortality (P=0.96), treatment failure (P=0.69), and overall mortality (P=0.62). There were no differences in the rate of recurrence of leukemia among the groups. There were no differences in outcome after cord-blood transplantation between patients with one HLA mismatch and those with two HLA mismatches.
Conclusions HLA-mismatched cord blood should be considered an acceptable source of hematopoietic stem-cell grafts for adults in the absence of an HLA-matched adult donor.
Van de tweede studie is hier het abstract ook uit British Journal of Medicin
Transplants of Umbilical-Cord Blood or Bone Marrow from Unrelated Donors in Adults with Acute Leukemia
Vanderson Rocha, M.D., Ph.D., Myriam Labopin, M.D., Guillermo Sanz, M.D., William Arcese, M.D., Rainer Schwerdtfeger, M.D., Alberto Bosi, M.D., Niels Jacobsen, M.D., Tapani Ruutu, M.D., Marcos de Lima, M.D., Jürgen Finke, M.D., Francesco Frassoni, M.D., Eliane Gluckman, M.D., for the Acute Leukemia Working Party of European Blood and Marrow Transplant Group and the Eurocord–Netcord Registry
ABSTRACT
Background Promising results of cord-blood transplants from unrelated donors have been reported in adults.
Methods We compared outcomes in 682 adults with acute leukemia who received a hematopoietic stem-cell transplant from an unrelated donor: 98 received cord blood and 584 received bone marrow. The transplantations were performed from 1998 through 2002 and reported to Eurocord and the European Blood and Marrow Transplant Group.
Results Recipients of cord blood were younger than recipients of bone marrow (median, 24.5 vs. 32 years of age; P<0.001), weighed less (median, 58 vs. 68 kg; P<0.001), and had more advanced disease at the time of transplantation (52 percent vs. 33 percent, P<0.001). All marrow transplants were HLA matched, whereas 94 percent of cord-blood grafts were HLA mismatched (P<0.001). The median number of nucleated cells that were infused was 0.23x108 per kilogram of the recipient's body weight for cord blood and 2.9x108 per kilogram for bone marrow (P<0.001). Multivariate analysis showed lower risks of grade II, III, or IV acute graft-versus-host disease (GVHD) after cord-blood transplantation (relative risk, 0.57; 95 percent confidence interval, 0.37 to 0.87; P=0.01), but neutrophil recovery was significantly delayed (relative risk, 0.49; 95 percent confidence interval, 0.41 to 0.58; P<0.001). The incidence of chronic GVHD, transplantation-related mortality, relapse rate, and leukemia-free survival were not significantly different in the two groups.
Conclusions Cord blood from an unrelated donor is an alternative source of hematopoietic stem cells for adults with acute leukemia who lack an HLA-matched bone marrow donor.
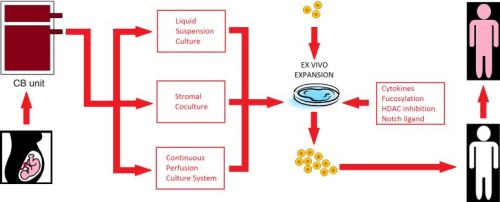
Current excitement in the field revolves around the development of safer techniques to improve homing, engraftment, and immune reconstitution after cord blood transplantation. Here the authors review the past, present, and future of cord transplantation.
Stem Cells Transl Med. 2014 Dec; 3(12): 1435–1443.
Concise Review: Umbilical Cord Blood Transplantation: Past, Present, and Future
Javier Munoz,
a,* Nina Shah,
 b,* Katayoun Rezvani
b,* Katayoun Rezvani,
b Chitra Hosing,
b Catherine M. Bollard,
c,d,e Betul Oran,
b Amanda Olson,
b Uday Popat,
b Jeffrey Molldrem,
b Ian K. McNiece,
b and
Elizabeth J. Shpallb
Abstract
Allogeneic hematopoietic stem cell transplantation is an important treatment option for fit patients with poor-risk hematological malignancies; nevertheless, the lack of available fully matched donors limits the extent of its use. Umbilical cord blood has emerged as an effective alternate source of hematopoietic stem cell support. Transplantation with cord blood allows for faster availability of frozen sample and avoids invasive procedures for donors. In addition, this procedure has demonstrated reduced relapse rates and similar overall survival when compared with unrelated allogeneic hematopoietic stem cell transplantation. The limited dose of CD34-positive stem cells available with single-unit cord transplantation has been addressed by the development of double-unit cord transplantation. In combination with improved conditioning regimens, double-unit cord transplantation has allowed for the treatment of larger children, as well as adult patients with hematological malignancies. Current excitement in the field revolves around the development of safer techniques to improve homing, engraftment, and immune reconstitution after cord blood transplantation. Here the authors review the past, present, and future of cord transplantation.
References:
1.
Ballen KK, King RJ, Chitphakdithai P, et al. The national marrow donor program 20 years of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14(suppl):2–7. [PubMed]2.
Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16:1541–1548. [PMC free article] [PubMed]3.
Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828–3832. [PMC free article] [PubMed]4.
Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. [PubMed]5.
Wagner JE, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: Analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795–802. [PubMed]6.
Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. [PubMed]7.
Barker JN, Krepski TP, DeFor TE, et al. Searching for unrelated donor hematopoietic stem cells: Availability and speed of umbilical cord blood versus bone marrow. Biol Blood Marrow Transplant. 2002;8:257–260. [PubMed]8.
Hakenberg P, Kögler G, Wernet P. NETCORD: A cord blood allocation network. Bone Marrow Transplant. 1998;22(suppl 1):S17–S18. [PubMed]9.
Petersdorf EW, Gooley TA, Anasetti C, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92:3515–3520. [PubMed]10.
Garderet L, Dulphy N, Douay C, et al. The umbilical cord blood alphabeta T-cell repertoire: Characteristics of a polyclonal and naive but completely formed repertoire. Blood. 1998;91:340–346. [PubMed]11.
Locatelli F, Rocha V, Chastang C, et al. Factors associated with outcome after cord blood transplantation in children with acute leukemia. Blood. 1999;93:3662–3671. [PubMed]12.
Michel G, Rocha V, Chevret S, et al. Unrelated cord blood transplantation for childhood acute myeloid leukemia: A Eurocord Group analysis. Blood. 2003;102:4290–4297. [PubMed]13.
Kurtzberg J, Prasad VK, Carter SL, et al. Results of the Cord Blood Transplantation Study (COBLT): Clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. [PMC free article] [PubMed]14.
Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: A comparison study. Lancet. 2007;369:1947–1954. [PubMed]15.
Fernandes JF, Rocha V, Labopin M, et al. Transplantation in patients with SCID: Mismatched related stem cells or unrelated cord blood? Blood. 2012;119:2949–2955. [PubMed]16.
Kamani NR, Walters MC, Carter S, et al. Unrelated donor cord blood transplantation for children with severe sickle cell disease: Results of one cohort from the phase II study from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Biol Blood Marrow Transplant. 2012;18:1265–1272. [PMC free article] [PubMed]17.
Escolar ML, Poe MD, Provenzale JM, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N Engl J Med. 2005;352:2069–2081. [PubMed]18.
Bhattacharya A, Slatter M, Curtis A, et al. Successful umbilical cord blood stem cell transplantation for chronic granulomatous disease. Bone Marrow Transplant. 2003;31:403–405. [PubMed]19.
Staba SL, Escolar ML, Poe M, et al. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med. 2004;350:1960–1969. [PubMed]20.
Boelens JJ, Aldenhoven M, Purtill D, et al. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood. 2013;121:3981–3987. [PMC free article] [PubMed]21.
Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. [PubMed]22.
Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. [PubMed]23.
Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. [PubMed]24.
Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. [PubMed]25.
Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: A retrospective analysis. Lancet Oncol. 2010;11:653–660. [PMC free article] [PubMed]26.
Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. [PubMed]27.
Ramirez P, Wagner JE, DeFor TE, et al. Factors predicting single-unit predominance after double umbilical cord blood transplantation. Bone Marrow Transplant. 2012;47:799–803. [PMC free article] [PubMed]28.
Gutman JA, Turtle CJ, Manley TJ, et al. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T-cell response against the nonengrafted unit. Blood. 2010;115:757–765. [PMC free article] [PubMed]29.
MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: Analysis of risk factors. Blood. 2009;113:2410–2415. [PMC free article] [PubMed]30.
Rodrigues CA, Sanz G, Brunstein CG, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: A study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27:256–263. [PubMed]31.
Kindwall-Keller TL, Hegerfeldt Y, Meyerson HJ, et al. Prospective study of one- vs two-unit umbilical cord blood transplantation following reduced intensity conditioning in adults with hematological malignancies. Bone Marrow Transplant. 2012;47:924–933. [PMC free article] [PubMed]32.
Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: Relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–4699. [PMC free article] [PubMed]33.
Barker JN, Weisdorf DJ, DeFor TE, et al. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–1919. [PubMed]34.
Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–89. [PMC free article] [PubMed]35.
Brunstein CG, Eapen M, Ahn KW, et al. Reduced-intensity conditioning transplantation in acute leukemia: The effect of source of unrelated donor stem cells on outcomes. Blood. 2012;119:5591–5598. [PMC free article] [PubMed]36.
Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: Results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. [PMC free article] [PubMed]37.
Wagner JE, Eapen M, Carter SL et al. No survival advantage after double umbilical cord blood (UCB) compared to single UCB transplant in children with hematological malignancy: Results of the blood and marrow transplant clinical trials network (BMT CTN 0501) randomized trial. Available at https://ash.confex.com/ash/2012/webprogram/Paper48413.html. Accessed September 2, 2014.38. Collins CL, Ahn KW, Wang Z et al. Long-term survival after alternative donor transplantation in children with acute leukemia. J Clin Oncol 2013;31(suppl):10006a.
39.
Dahi PB, Ponce DM, Byam C et al. Prospective evaluation of alternative donor availability in 708 patients: Improved allograft access with enlarging CB inventory for all patients including racial and ethnic minorities. Available at https://ash.confex.com/ash/2013/webprogram/Paper60210.html. Accessed September 2, 2014.40.
Bachanova V, Brunstein C, Burns LJ et al. Alternative donor transplantation for adults with lymphoma: Comparison of umbilical cord blood versus 8/8 HLA-matched donor (URD) versus 7/8 URD. Available at https://ash.confex.com/ash/2013/webprogram/Paper57320.html. Accessed September 2, 2014.41.
Stiff PJ, Montesinos P, Peled T et al. StemEx (copper chelation based) ex vivo expanded umbilical cord blood stem cell transplantation (UCBT) accelerates engraftment and improves 100 day survival in myeloablated patients compared to a registry cohort undergoing double unit UCBT: Results of a multicenter study of 101 patients with hematologic malignancies. Available at https://ash.confex.com/ash/2013/webprogram/Paper61421.html. Accessed September 2, 2014.42.
Vasileiou S, Baltadakis I, Panitsas F et al. Comparative analysis of cell dose and viability of cord blood units at cryopreservation and at thaw/infusion for unrelated stem cell transplantation in adult recipients. Available at https://bmt.confex.com/tandem/2014/webprogram/Paper4069.html. Accessed September 2, 2014.43.
Ponce DM, Hilden P, Mumaw C et al. High day 28 ST2 biomarker levels predict severe day 100 acute graft-versus-host disease and day 180 transplant-related mortality after double-unit cord blood transplantation. Available at https://ash.confex.com/ash/2013/webprogram/Paper60713.html. Accessed September 2, 2014. [PubMed]44. Poon ML, Linn YC, Lim Z, et al. Double unit umbilical cord blood transplant for adults with acute leukemia and myelodysplastic syndrome results in comparable outcome as matched sibling or unrelated donor transplant only after myeloablative conditioning but not reduced intensity conditio. Biol Blood Marrow Transplant. 2014;20(suppl):S247.
45. Ghantoji SS, Goddu S, Sreenivasula S, et al. Characteristics and outcome of cytomegalovirus (CMV) infections in 95 cord blood transplant (CBT) recipients: The MD Anderson experience. Biol Blood Marrow Transplant. 2014;20(suppl):S223–S224.
46. Barker JN, Ponce DM, Dahi P, et al. Double-unit cord blood (CB) transplantation combined with haplo-identical CD34+ cells results in 100% CB engraftment with enhanced myeloid CD34+ recovery. Biol Blood Marrow Transplant. 2014;20(suppl):S138–S139.
47. Volodin L, Beitinjaneh A, Salman H, et al. Outcomes of double unit umbilical cord blood transplantation using fludarabine/busulfan based reduced intensity conditioning regimen. Biol Blood Marrow Transplant. 2014;20(suppl):S142.
48. Ponce DM, Devlin S, Lubin M, et al. High disease-free survival and enhanced protection against relapse after double-unit cord blood transplantation (DCB-T) when compared to unrelated donor transplantation (URD-T) in patients with acute leukemia, MDS and CML. Biol Blood Marrow Transplant. 2014;20(suppl):S58–S59.
50.
Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357:1472–1475. [PubMed]51.
Oran B, Shpall E. Umbilical cord blood transplantation: A maturing technology. Hematology (Am Soc Hematol Educ Program) 2012;2012:215–222. [PubMed]52.
de Lima M, McMannis J, Gee A, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: A phase I/II clinical trial. Bone Marrow Transplant. 2008;41:771–778. [PMC free article] [PubMed]53.
Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–236. [PMC free article] [PubMed]54.
Robinson SN, Ng J, Niu T, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–366. [PMC free article] [PubMed]55.
Kelly SS, Sola CB, de Lima M, et al. Ex vivo expansion of cord blood. Bone Marrow Transplant. 2009;44:673–681. [PMC free article] [PubMed]56.
de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367:2305–2315. [PMC free article] [PubMed]57.
Frassoni F, Gualandi F, Podestà M, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: A phase I/II study. Lancet Oncol. 2008;9:831–839. [PubMed]58.
Bautista G, Cabrera JR, Regidor C, et al. Cord blood transplants supported by co-infusion of mobilized hematopoietic stem cells from a third-party donor. Bone Marrow Transplant. 2009;43:365–373. [PubMed]59.
Fernández MN, Regidor C, Cabrera R, et al. Unrelated umbilical cord blood transplants in adults: Early recovery of neutrophils by supportive co-transplantation of a low number of highly purified peripheral blood CD34+ cells from an HLA-haploidentical donor. Exp Hematol. 2003;31:535–544. [PubMed]60.
Xia L, McDaniel JM, Yago T, et al. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood. 2004;104:3091–3096. [PubMed]61.
Robinson SN, Simmons PJ, Thomas MW, et al. Ex vivo fucosylation improves human cord blood engraftment in NOD-SCID IL-2Rγ(null) mice. Exp Hematol. 2012;40:445–456. [PMC free article] [PubMed]62.
Hoggatt J, Singh P, Sampath J, et al. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. [PMC free article] [PubMed]63.
Cutler C, Multani P, Robbins D, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122:3074–3081. [PMC free article] [PubMed]64.
Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood. 2011;117:1061–1070. [PMC free article] [PubMed]65.
Shah N, Martin-Antonio B, Yang H, et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS One. 2013;8:e76781. [PMC free article] [PubMed]66.
Hanley PJ, Cruz CR, Savoldo B, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114:1958–1967. [PMC free article] [PubMed]67.
Jain N, Liu H, Artz AS, et al. Immune reconstitution after combined haploidentical and umbilical cord blood transplant. Leuk Lymphoma. 2013;54:1242–1249. [PubMed]69.
Gluckman E, Ruggeri A, Volt F, et al. Milestones in umbilical cord blood transplantation. Br J Haematol. 2011;154:441–447. [PubMed]70.
Cooper B. The origins of bone marrow as the seedbed of our blood: From antiquity to the time of Osler. Proc (Bayl Univ Med Cent) 2011;24:115–118. [PMC free article] [PubMed]71. Osgood EE, Riddle MC, Mathews TJ. Aplastic anemia treated with daily transfusions and intravenous marrow: Case report. Ann Intern Med. 1939;13:357–367.
72.
Congdon CC. Experimental treatment of total-body irradiation injury: A brief review. Blood. 1957;12:746–754. [PubMed]73.
Thomas ED, Lochte HL, Jr, Cannon JH, et al. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest. 1959;38:1709–1716. [PMC free article] [PubMed]74.
Thomas ED, Lochte HL, Jr, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491–496. [PubMed]75.
Thomas ED, Lochte HL, Jr, Ferrebee JW. Irradiation of the entire body and marrow transplantation: Some observations and comments. Blood. 1959;14:1–23. [PubMed]76.
Thomas E, Storb R, Clift RA, et al. Bone-marrow transplantation (first of two parts) N Engl J Med. 1975;292:832–843. [PubMed]77.
Gatti RA, Meuwissen HJ, Allen HD, et al. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–1369. [PubMed]78.
Graw RG, Jr, Lohrmann HP, Bull MI, et al. Bone-marrow transplantation following combination chemotherapy immunosuppression (B.A.C.T.) in patients with acute leukemia. Transplant Proc. 1974;6:349–354. [PubMed]79.
Bleyer WA, Blaese RM, Bujak JS, et al. Long-term remission from acute myelogenous leukemia after bone marrow transplantation and recovery from acute graft-versus-host reaction and prolonged immunoincompetence. Blood. 1975;45:171–181. [PubMed]80.
Appelbaum FR, Herzig GP, Ziegler JL, et al. Successful engraftment of cryopreserved autologous bone marrow in patients with malignant lymphoma. Blood. 1978;52:85–95. [PubMed]81.
Thomas ED. The Nobel Lectures in Immunology: The Nobel Prize for Physiology or Medicine, 1990. Bone marrow transplantation: Past, present and future. Scand J Immunol. 1994;39:339–345. [PubMed]82.
Laporte JP, Gorin NC, Rubinstein P, et al. Cord-blood transplantation from an unrelated donor in an adult with chronic myelogenous leukemia. N Engl J Med. 1996;335:167–170. [PubMed]83.
Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. N Engl J Med. 1997;337:373–381. [PubMed]84.
Rocha V, Wagner JE, Jr, Sobocinski KA, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. N Engl J Med. 2000;342:1846–1854. [PubMed]85.
Cairo MS, Wagner JE. Placental and/or umbilical cord blood: An alternative source of hematopoietic stem cells for transplantation. Blood. 1997;90:4665–4678. [PubMed]86.
Sanz GF, Saavedra S, Planelles D, et al. Standardized, unrelated donor cord blood transplantation in adults with hematologic malignancies. Blood. 2001;98:2332–2338. [PubMed]87.
Shpall EJ, Quinones R, Giller R, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8:368–376. [PubMed]88.
Kögler G, Sensken S, Airey JA, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. [PMC free article] [PubMed]89.
Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: Impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. [PMC free article] [PubMed]90.
Rocha V, Crotta A, Ruggeri A, et al. Double cord blood transplantation: Extending the use of unrelated umbilical cord blood cells for patients with hematological diseases. Best Pract Res Clin Haematol. 2010;23:223–229. [PubMed]91.
Robinson SN, Thomas MW, Simmons PJ, et al. Fucosylation with fucosyltransferase VI or fucosyltransferase VII improves cord blood engraftment. Cytotherapy. 2014;16:84–89. [PMC free article] [PubMed]92.
Durand B, Eddleman K, Migliaccio AR, et al. Long-term generation of colony-forming cells (CFC) from CD34+ human umbilical cord blood cells. Leuk Lymphoma. 1993;11:263–273. [PubMed]93.
Kurata H, Takakuwa K, Tanaka K. Ex vivo expansion of hematopoietic progenitor cells in human cord blood: An effect enhanced by cord blood serum. Hematol Pathol. 1995;9:73–78. [PubMed]94.
Bertolini F, Soligo D, Lazzari L, et al. The effect of interleukin-12 in ex-vivo expansion of human haemopoietic progenitors. Br J Haematol. 1995;90:935–938. [PubMed]95.
Siena S, Di Nicola M, Bregni M, et al. Massive ex vivo generation of functional dendritic cells from mobilized CD34+ blood progenitors for anticancer therapy. Exp Hematol. 1995;23:1463–1471. [PubMed]96.
DiGiusto DL, Lee R, Moon J, et al. Hematopoietic potential of cryopreserved and ex vivo manipulated umbilical cord blood progenitor cells evaluated in vitro and in vivo. Blood. 1996;87:1261–1271. [PubMed]97.
Scaradavou A, Isola L, Rubinstein P, et al. A murine model for human cord blood transplantation: Near-term fetal and neonatal peripheral blood cells can achieve long-term bone marrow engraftment in sublethally irradiated adult recipients. Blood. 1997;89:1089–1099. [PubMed]98.
Ohmizono Y, Sakabe H, Kimura T, et al. Thrombopoietin augments ex vivo expansion of human cord blood-derived hematopoietic progenitors in combination with stem cell factor and flt3 ligand. Leukemia. 1997;11:524–530. [PubMed]99.
De Bruyn C, Delforge A, Bron D, et al. Ex vivo expansion of CD34 + CD38- cord blood cells. J Hematother. 1997;6:93–102. [PubMed]100.
Piacibello W, Sanavio F, Garetto L, et al. Extensive amplification and self-renewal of human primitive hematopoietic stem cells from cord blood. Blood. 1997;89:2644–2653. [PubMed]101.
Kögler G, Callejas J, Sorg RV, et al. The effect of different thawing methods, growth factor combinations and media on the ex vivo expansion of umbilical cord blood primitive and committed progenitors. Bone Marrow Transplant. 1998;21:233–241. [PubMed]102.
Kögler G, Callejas J, Sorg RV, et al. An eight-fold ex vivo expansion of long-term culture-initiating cells from umbilical cord blood in stirred suspension cultures. Bone Marrow Transplant. 1998;21(suppl 3):S48–S53. [PubMed]103.
Köhler T, Plettig R, Wetzstein W, et al. Defining optimum conditions for the ex vivo expansion of human umbilical cord blood cells. Influences of progenitor enrichment, interference with feeder layers, early-acting cytokines and agitation of culture vessels. Stem Cells. 1999;17:19–24. [PubMed]104.
Nakamura Y, Ando K, Chargui J, et al. Ex vivo generation of CD34(+) cells from CD34(−) hematopoietic cells. Blood. 1999;94:4053–4059. [PubMed]105.
McNiece I, Kubegov D, Kerzic P, et al. Increased expansion and differentiation of cord blood products using a two-step expansion culture. Exp Hematol. 2000;28:1181–1186. [PubMed]106.
Lewis ID, Almeida-Porada G, Du J, et al. Umbilical cord blood cells capable of engrafting in primary, secondary, and tertiary xenogeneic hosts are preserved after ex vivo culture in a noncontact system. Blood. 2001;97:3441–3449. [PubMed]107.
Pecora AL, Stiff P, Jennis A, et al. Prompt and durable engraftment in two older adult patients with high risk chronic myelogenous leukemia (CML) using ex vivo expanded and unmanipulated unrelated umbilical cord blood. Bone Marrow Transplant. 2000;25:797–799. [PubMed]108.
Broxmeyer HE, Srour EF, Hangoc G, et al. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc Natl Acad Sci USA. 2003;100:645–650. [PMC free article] [PubMed]109.
Jaroscak J, Goltry K, Smith A, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: Results of a phase 1 trial using the AastromReplicell System. Blood. 2003;101:5061–5067. [PubMed]110.
Peled T, Mandel J, Goudsmid RN, et al. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy. 2004;6:344–355. [PubMed]111.
Serrano LM, Pfeiffer T, Olivares S, et al. Differentiation of naive cord-blood T cells into CD19-specific cytolytic effectors for posttransplantation adoptive immunotherapy. Blood. 2006;107:2643–2652. [PMC free article] [PubMed]112.
Chaurasia P, Gajzer DC, Schaniel C, et al. Epigenetic reprogramming induces the expansion of cord blood stem cells. J Clin Invest. 2014;124:2378–2395. [PMC free article] [PubMed]113.
Bart T, Boo M, Balabanova S, et al. Impact of selection of cord blood units from the United States and swiss registries on the cost of banking operations. Transfus Med Hemother. 2013;40:14–20. [PMC free article] [PubMed]114.
Delaney C, Bollard CM, Shpall EJ. Cord blood graft engineering. Biol Blood Marrow Transplant. 2013;19(suppl):S74–S78. [PMC free article] [PubMed]
stamceltransplantaties, leukemie, navelstrengbloed
Gerelateerde artikelen





 b,*
b,*
Plaats een reactie ...
Reageer op "Navelstrengbloed werkt bij leukemie goed en soms genezend, als er geen goede donor is voor stamceltransplantatie of beenmergtransplantatie blijkt uit meerdere studies"