Helpt u ons aan 500 donateurs?
19 april 2018: lees ook dit artikel:
1 maart 2018: Bron:
- Nature Communicationsvolume 9, Article number: 896 (2018)
- doi:10.1038/s41467-018-03215-x
Een bloedtest kan aan de hand van in bloed circulerende DNA al binnen twee tot drie weken bepalen of palbociclip effectief is bij patiënten met borstkanker.
Bewijst een nieuwe studie: Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer
De onderzoekers testten vrouwen met oestrogeenreceptor-positieve borstkanker - de meest voorkomende soort - die deelnamen aan een klinische studie met palbociclib voor gevorderde borstkanker.
Momenteel moeten vrouwen twee tot drie maanden wachten om erachter te komen, met behulp van een scan, of palbociclib werkt.
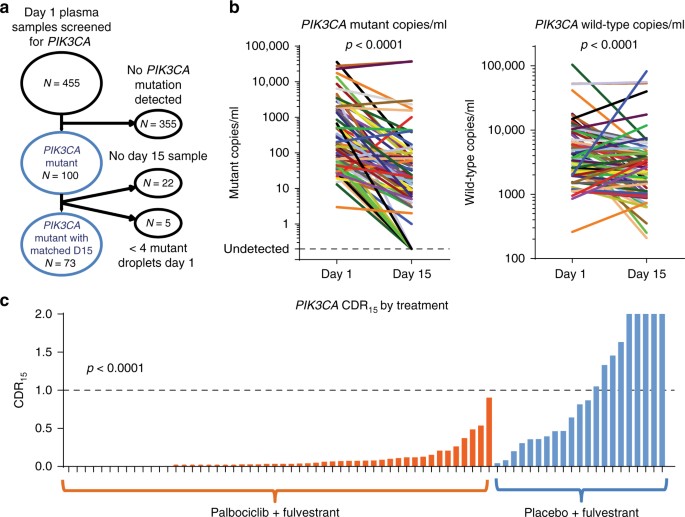
De nieuwe bloedtest zoekt in plaats daarvan naar circulerend tumor-DNA - fragmenten van DNA die zijn afgestoten door de kanker die in de bloedbaan is terechtgekomen.
De DNA-mutaties die gerelateerd zijn aan de kankercellen kunnen in deze monsters worden gedetecteerd.
De onderzoekers ontdekten dat ze konden voorspellen of de behandeling met palbociclib zou werken door de hoeveelheid van een PIK3CA-gen te vergelijken die werd gedetecteerd in een bloedtest vóór de behandeling en 15 dagen na het begin van de behandeling.
In de studie hadden 73 vrouwen de PIK3CA-mutatie en kregen ze bloedtesten voor en na het starten van de palbociclib-behandeling.
Bij deze vrouwen ontdekten de onderzoekers dat degenen die na 15 dagen een kleine afname in PIK3CA-circulerend DNA hadden, een mediane progressievrije overleving hadden (de tijd dat de patiënt overleefde en de kanker niet erger werd) van slechts 4,1 maanden, vergeleken met bij vrouwen met een grote afname van PIK3CA, met een mediane progressievrije overleving van 11,2 maanden.
Opnieuw dus een bewijs dat via circulerend DNA in bloed is vast te stellen of een behandeling werkt. Al die dure medijnen hoeven dus niet zo heel lan g gebruikt te worden voordat aangetoond kan wroden of ze ook werken. Zie het volledige studierapport voor alle details
13 september 2017: Bron: J Glob Oncol. 2017 Aug; 3(4): 289–303. Published online 2017 Apr 11
Palbociclib samen met fulvestrand geeft verdubbeling van progressievrije ziekte en langere overall overleving in vergelijking met alleen fulvestrand bij hormoonresistente borstkanker. Ook bij Aziatische vrouwen blijken die resultaten overeen te komen met niet-Azoatische vrouwen, zowel voor als na de overgang . Dit bljikt uit een vooraf geplande placebo gecontroleerde gerandomiseerde subgroep binnen de PALOMA-3 studie. De resultaten uit de PALOMA-3 studie bij vrouwen met hormoonresistente borstkanker gaf een verdubbeling van progressievrije tijd te zien (4,6 vs 9,5 maanden) (zie abstract onderaan artikel. En is vergelijkbaar met een studie analyse bij een groep Aziatische vrouwen.
Deze groep van vrouwen waren allemaal van Aziatische afkomst, zowel premenopauzale en postmenopauzale. Zij hadden allemaal palbociclib plus fulvestrant (n = 71) of placebo plus fulvestrant (n = 31) gekregen als behandeling. Palbociclib plus fulvestrant verbeterde de progressie-vrije overleving (PFS) in vergelijking met fulvestrant alleen. Mediane PFS werd tot nu toe niet bereikt met palbociclib plus fulvestrant (95% CI, 9,2 maanden niet bereikt), maar was 5,8 maanden met placebo plus fulvestrant (95% CI, 3,5 tot 9,2 maanden, relatieve risicoi, 0,485, 95% CI, 0,270 tot 0,869 ; P = .0065).
Zie grafiek om vergeljiking te maken met niet-Aziatische vrouwen
Tekst gaat verder onder grafiek:
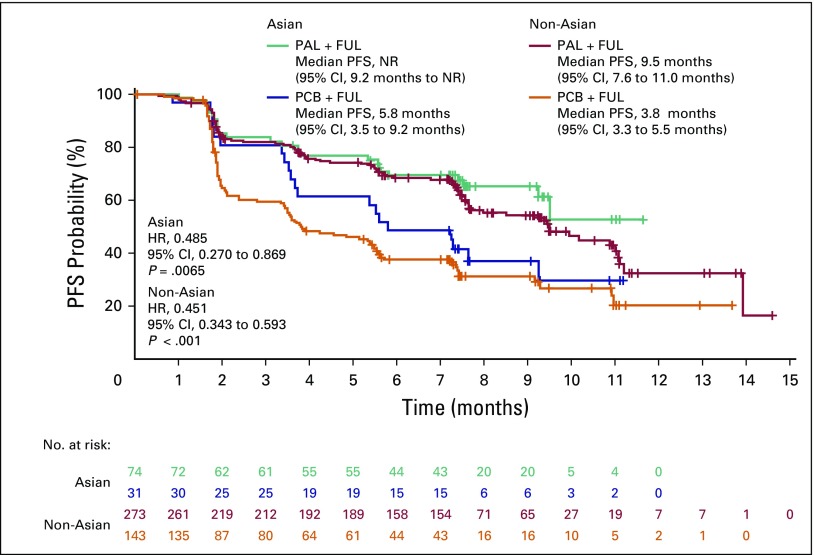
De meest voorkomende graad 3 of 4 bijwerkingen in de palbociclibgroep waren neutropenie (92%) en leukopenie (29%); Febriele neutropenie bij 4,1% van de patiënten. Deze waren vergelijkbaar met de bijwerkingen bij niet-Aziatische vrouwen die palcociclib kregen.
Zie bijwerkingengrafiek:

Conclusie:
Dit is het eerste studierapport, naar ons weten, dat Palbociclib plus fulvestrant PFS bij Aziatische patiënten verbetert. Palbociclib plus fulvestrant werd goed verdragen in deze studie.
Het volledige studierapport: PALOMA-3: Phase III Trial of Fulvestrant With or Without Palbociclib in Premenopausal and Postmenopausal Women With Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer That Progressed on Prior Endocrine Therapy—Safety and Efficacy in Asian Patients is gratis in te zien.
Hier het abstract van de studies:
Palbociclib plus fulvestrant treatment was well-tolerated, and the primary toxicity of asymptomatic neutropenia was effectively managed by dose modification without apparent loss of efficacy.
Source: The oncologist
The Oncologist October 2016 vol. 21 no. 10 1165-1175
Palbociclib in Combination With Fulvestrant in Women With Hormone Receptor-Positive/HER2-Negative Advanced Metastatic Breast Cancer: Detailed Safety Analysis From a Multicenter, Randomized, Placebo-Controlled, Phase III Study (PALOMA-3)
Sunil Vermaa, Cynthia Huang Bartlettb, Patrick Schnellc, Angela M. DeMicheled, Sherene Loie, Jungsil Rof, Marco Colleonig, Hiroji Iwatah, Nadia Harbecki, Massimo Cristofanillij, Ke Zhangk, Alexandra Thielel, Nicholas C. Turnerm and Hope S. Rugon + Author Affiliations aUniversity of Calgary, Alberta, Canada bPfizer Inc., Collegeville, Pennsylvania, USA cPfizer Inc., New York, New York, USA dUniversity of Pennsylvania, Philadelphia, Pennsylvania, USA ePeter MacCallum Cancer Centre, East Melbourne, Victoria, Australia fNational Cancer Center, Goyang-si, Republic of Korea gIstituto Europeo di Oncologia, Milan, Italy hAichi Cancer Center Hospital, Nagoya, Japan iBrustzentrum der Universität München, Munich, Germany jRobert H. Lurie Comprehensive Cancer Center, Feinberg School of Medicine, Chicago, Illinois, USA kPfizer Inc., San Diego, California, USA lPfizer Inc., Cambridge, Massachusetts, USA mInstitute of Cancer Research and Royal Marsden Hospital, London, United Kingdom nUniversity of California San Francisco Helen Diller Family Comprehensive Cancer Center, San Francisco, California, USA. Correspondence: Sunil Verma, M.D., Department of Oncology, Tom Baker Cancer Centre, University of Calgary, 1331 29th Street NW, Calgary, Alberta T2N 4N2, Canada. Telephone: 403-521-3701; E-Mail: DrSunil.Verma@ahs.ca
Received March 9, 2016. Accepted May 10, 2016. Published online before print July 1, 2016.
Disclosures of potential conflicts of interest may be found at the end of this article. Abstract Background. Palbociclib enhances endocrine therapy and improves clinical outcomes in hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative metastatic breast cancer (MBC). Because this is a new target, it is clinically important to understand palbociclib’s safety profile to effectively manage toxicity and optimize clinical benefit.
Materials and Methods.
Patients with endocrine-resistant, HR-positive/HER2-negative MBC (n = 521) were randomly assigned 2:1 to receive fulvestrant (500 mg intramuscular injection) with or without goserelin with oral palbociclib (125 mg daily; 3 weeks on/1 week off) or placebo. Safety assessments at baseline and day 1 of each cycle included blood counts on day 15 for the first 2 cycles. Hematologic toxicity was assessed by using laboratory data.
Results.
A total of 517 patients were treated (palbociclib, n = 345; placebo, n = 172); median follow-up was 8.9 months. With palbociclib, neutropenia was the most common grade 3 (55%) and 4 (10%) adverse event; median times to onset and duration of grade ≥3 episodes were 16 and 7 days, respectively. Asian ethnicity and below-median neutrophil counts at baseline were significantly associated with an increased chance of developing grade 3–4 neutropenia with palbociclib. Dose modifications for grade 3–4 neutropenia had no adverse effect on progression-free survival. In the palbociclib arm, febrile neutropenia occurred in 3 (<1%) patients. The percentage of grade 1–2 infections was higher than in the placebo arm. Grade 1 stomatitis occurred in 8% of patients.
Conclusion.
Palbociclib plus fulvestrant treatment was well-tolerated, and the primary toxicity of asymptomatic neutropenia was effectively managed by dose modification without apparent loss of efficacy. This study appears at ClinicalTrials.gov, NCT01942135. Implications for Practice: Treatment with palbociclib in combination with fulvestrant was generally safe and well-tolerated in patients with hormone receptor (HR)-positive metastatic breast cancer. Consistent with the drug's proposed mechanism of action, palbociclib-related neutropenia differs in its clinical time course, patterns, and consequences from those seen with chemotherapy. Neutropenia can be effectively managed by a dose reduction, interruption, or cycle delay without compromising efficacy. A significant efficacy gain and a favorable safety profile support the consideration of incorporating palbociclib into the routine management of HR-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer.
This is the first report, to our knowledge, showing that palbociclib plus fulvestrant improves PFS in asian patients. Palbociclib plus fulvestrant was well tolerated in this study.
PALOMA-3: Phase III Trial of Fulvestrant With or Without Palbociclib in Premenopausal and Postmenopausal Women With Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer That Progressed on Prior Endocrine Therapy—Safety and Efficacy in Asian Patients
Hiroji Iwata, Seock-Ah Im, Norikazu Masuda, Young-Hyuck Im, Kenichi Inoue, Yoshiaki Rai, Rikiya Nakamura, Jee Hyun Kim, Justin T. Hoffman, Ke Zhang, Carla Giorgetti, Shrividya Iyer, Patrick T. Schnell, Cynthia Huang Bartlett, and Jungsil Ro
Seock-Ah Im, Norikazu Masuda, Young-Hyuck Im, Kenichi Inoue, Yoshiaki Rai, Rikiya Nakamura, Jee Hyun Kim, Justin T. Hoffman, Ke Zhang, Carla Giorgetti, Shrividya Iyer, Patrick T. Schnell, Cynthia Huang Bartlett, and Jungsil Ro
Abstract
Purpose
To assess efficacy and safety of palbociclib plus fulvestrant in Asians with endocrine therapy–resistant metastatic breast cancer.
Patients and Methods
The Palbociclib Ongoing Trials in the Management of Breast Cancer 3 (PALOMA-3) trial, a double-blind phase III study, included 521 patients with hormone receptor–positive/human epidermal growth factor receptor 2–negative metastatic breast cancer with disease progression on endocrine therapy. Patient-reported outcomes (PROs) were assessed on study treatment and at the end of treatment.
Results
This preplanned subgroup analysis of the PALOMA-3 study included premenopausal and postmenopausal Asians taking palbociclib plus fulvestrant (n = 71) or placebo plus fulvestrant (n = 31). Palbociclib plus fulvestrant improved progression-free survival (PFS) compared with fulvestrant alone. Median PFS was not reached with palbociclib plus fulvestrant (95% CI, 9.2 months to not reached) but was 5.8 months with placebo plus fulvestrant (95% CI, 3.5 to 9.2 months; hazard ratio, 0.485; 95% CI, 0.270 to 0.869; P = .0065). The most common all-cause grade 3 or 4 adverse events in the palbociclib arm were neutropenia (92%) and leukopenia (29%); febrile neutropenia occurred in 4.1% of patients. Within-patient mean trough concentration comparisons across subgroups indicated similar palbociclib exposure between Asians and non-Asians. Global quality of life was maintained; no statistically significant changes from baseline were observed for patient-reported outcome scores with palbociclib plus fulvestrant.
Conclusion
This is the first report, to our knowledge, showing that palbociclib plus fulvestrant improves PFS in asian patients. Palbociclib plus fulvestrant was well tolerated in this study.
REFERENCES
Gerelateerde artikelen
- Borstkanker: Palbociclib, gegeven naast letrozole - femara verdubbelt ziektevrije tijd bij borstkanker ER-pos en Her2-neg copy 1
- Borstkanker: Pertuzumab - Perjeta door FDA goedgekeurd als eerstelijns behandeling immuuntherapie bij operabele borstkanker HER-2 positief na uitstekende resultaten uit fase 3 APHINITY studie copy 1



Plaats een reactie ...
Reageer op "Borstkanker: Palpociclib plus fulvestrand geeft langere overall overleving en progressievrije ziekte dan alleen fulvesrtrand bij hormoonresistente borstkanker"