Helpt u ons aan 500 donateurs om kanker-actueel online te kunnen houden?
1 november 2018: Zie ook dit artikel:
1 augustus 2018: lees ook dit artikel:
4 april 2018: lees ook dit artikel:
en zie ook de artikelen in gerelateerde artikelen
4 april 2018: Bron: HOPA 2018
Rucaparib (RUBRACA) een zogeheten parpremmer verlengt als onderhoudsbehandeling de progressievrije overleving met meer dan 100 procent in vergelijking met een placebo bij vrouwen met een recidief van platinum-gevoelige (chemo gevoelig) eierstokkanker. De ziektevrije en progressievrije overleving in vergelijking met een placebo was beduidend beter voor rucaparib, 13,6 maanden vs 5,4 voor placebo bij alle deelnemende patiënten ongeacht hun BRCA status. Dus ook bij vrouwen met eierstokkanker die geen BRCA mutatie hebben zorgt rucaparib (RUBRACA) voor uitstekende resultaten.
Bij vrouwen met wel een BRCA mutatie status waren de resultaten nog beter, 16,6 maanden voor rucaparib versus 5,4 maanden voor placebo. Blijkt uit de ARIEL3 fase III studie.
Hier een grafiek van hoe de ARIEL studies zijn verlopen afgelopen jaren (tekst gaat verder onder grafiek) :
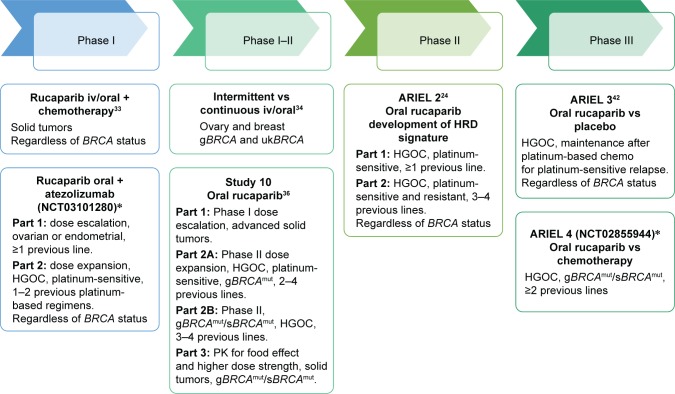
Bovenstaande en onderstaande grafiek komt uit een reviewstudie december 2017 over rucaparuib bij zowel eierstokkanker als borstkanker: Emerging treatment options for ovarian cancer: focus on rucaparib
Table 3
PFS/ORR by HRD sub-group with rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 part 1)5
| HRD subgroups of high-grade ovarian cancer | Number of patients in each group | Median PFS, months (95% CI) | Hazard ratio | ORR | |
|---|---|---|---|---|---|
| RECIST, % (n, 95% CI) | RECIST/CA125, % (n, 95% CI) | ||||
| BRCA mutant | 40 | 12.8 (9.0–14.7) | 0.27, P<0.0001 | 80% (32, 64–91) | 85% (34, 70–94) |
| BRCA wild type and LOH high | 82 | 5.7 (5.3–7.6) | 0.62, P<0.011 | 29% (24, 20–40) | 44% (34, 33–55) |
| BRCA wild type and LOH low | 70 | 5.2 (3.6–5.5) | – | 10% (7, 4–20) | 20% (14, 11–31) |
Abbreviations: BRCA, breast and ovarian cancer susceptibility gene; CI, confidence interval; CA125, cancer antigen 125; HRD, homologous recombination repair deficiency; LOH, loss of heterozygosity; ORR, objective response rate; PFS, progression-free survival; RECIST, Response Evaluation Criteria In Solid Tumors (Version 1.1).
Uit de presentatie van de nieuwste studie op HOPA 2018:
Op basis van een intent-to-treat analyse was de mediane progressievrije overleving 10,8 maanden (95% betrouwbaarheidsinterval 8,3 - 11,4) met rucaparib, vergeleken met 5,4 maanden (95% betrouwbaarheidsinterval 5,3 - 5,5) voor placebo (P <.0001 ).
Van de 354 homologe recombinatie-deficiënte patiënten was de mediane progressievrije overleving met rucaparib 13,6 maanden (95% betrouwbaarheidsinterval 10,9 - 16,2) en 5,4 maanden voor placebo (95% betrouwbaarheidsinterval 5,1 - 5,6) (p <.0001).
Bij de 196 patiënten, specifiek met BRCA-mutaties, was de mediane progressievrije overleving voor rucaparib 16,6 maanden (95% betrouwbaarheidsinterval 13,4 - 22,9) en 5,4 maanden voor placebo (95% betrouwbaarheidsinterval 3,4 - 6,7) (P <.0001).
De meest gemelde aan de behandeling gerelateerde bijwerkingen waren misselijkheid en braken, evenals asthenie en bloedarmoede. De auteurs melden dat deze consistent zijn met eerdere studies van rucaparib. De bijwerkingen werden behandeld met profylactische en / of ondersteunende zorg, onderbrekingen in de behandeling en / of dosisverlagingen.
Conclusie:
Rucaparib effectively increases progression-free survival with high-grade, recurrent, platinum-sensitive ovarian cancer regardless of HRR status. They suggest that it could be considered as a new standard of care for women with platinum-sensitive ovarian cancer following a partial or complete response to second-line or later platinum-based chemotherapy.
Het volledige studierapport van de HOPA gepresenteerde studie is in december 2017 gepubliceerd in The Lancet: Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial en tegen betaling in te zien.
Hier het abstract van de studie met referentielijst van reviewstudie:
Rucaparib effectively increases progression-free survival with high-grade, recurrent, platinum-sensitive ovarian cancer regardless of HRR status. They suggest that it could be considered as a new standard of care for women with platinum-sensitive ovarian cancer following a partial or complete response to second-line or later platinum-based chemotherapy.
Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial
Plaats een reactie ...
1 Reactie op "Parpremmer rucaparib verdubbelt ziektevrije overleving (5 vs 11 en 13 maanden) bij chemo gevoelige eierstokkanker. Ook bij patienten zonder BRCA mutatie is rucaparib effectief"
Gerelateerde artikelen
- Niraparib - Zejula als onderhoudsbehandeling bij gevorderde eierstokkanker geeft een veel langere progressievrije overleving bij zowel patiënten met BRCA-gemuteerde eierstokkanker als bij niet BRCA-gemuteerd
- niraparib plus bevacizumab (Avastin) zonder chemo of gegeven in chemovrije periode verdubbelt bij eierstokkanker ziektevrije tijd en ziektevrije overleving steeg met 26 procent (79 vs 53 procent)
- Niraparib geeft zeer goede resultaten bij recidief van gevorderde eierstokkanker die eerder gevoelig bleek voor op platinum gebaseerde chemo copy 1
- Parpremmers Olaparib en niraparib gegeven als onderhoudsbehandeling bij gevorderde chemo gevoelige eierstokkanker heeft geen negatief effect op kwaliteit van leven en geeft wel betere overall overleving
- PARP remmers zoals olaparib zouden in vroeger stadium van eierstokkanker en andere vormen van kanker met BRCA mutaties moeten worden ingezet want teveel chemokuren verminderen kans op aanslaan van de behandeling, stellen oncologen n.a.v. diverse studies
- Talazoparib, een PARP remmer, wordt in veel studies bij veel verschillende vormen van kanker en in combinatie met andere medicijnen onderzocht en geeft veelbelovende resultaten copy 1
- Olaparib plus Bevacizumab als eerstelijns onderhoudsbehandeling voor eierstokkanker geeft betere progressievrije ziekte dan placebo ongeacht BRCA status.
- Olaparib als onderhoudsbehandeling voor BRCA 1/2 uitgezaaide platinum gevoelige eierstokkanker geeft 70 procent minder kans op overlijden in vergelijking met placebo
- Olaparib, een PARP remmer, verlengt ziektevrije en overall overleving in vergelijking met placebo bij eierstokkanker met BRCA 1 en 2
- Olaparib plus cedinarib lijkt doorbraak bij controle van vergevorderde eierstokkanker en verdubbelt progressievrije overleving van 9,2 maanden naar 17,7 maanden
- Parpremmer niraparib naast chemo en daarna als onderhoudsbehandeling verdubbelt mediane overall overleving in vergelijking met placebo
- Parpremmer Veliparib naast chemo gevolgd door veliparib alleen als onderhoudsbehandeling geeft betere overall overleving voor patienten met eierstokkanker stadium III en IV.
- Patienten met gevorderde eierstokkanker met een PARP-7 mutatie / expressie blijken veel betere mediane overall overleving te hebben (45 vs 16 maanden) dan zonder PARP-7 mutatie / expressie. copy 1
- Parpremmer rucaparib verdubbelt ziektevrije overleving (5 vs 11 en 13 maanden) bij chemo gevoelige eierstokkanker. Ook bij patienten zonder BRCA mutatie is rucaparib effectief
- PARP remmers zoals olaparib zouden in vroeger stadium van eierstokkanker en andere vormen van kanker met BRCA mutaties moeten worden ingezet
- BRCA - erfelijkheid: Combinatie van sapacitabine en seliciclib geeft een hoopvol therapeutisch effect bij zwaar voorbehandelde kankerpatienten met solide tumoren met onderliggende afwijkende erfelijke BCRA gen mutaties. copy 1
- PARP remmers zoals olaparib, niraparib en rucaparib zijn effectief zowel met als zonder BRCA mutaties, een overzicht




 ARIEL3 investigators†
ARIEL3 investigators†
Ook de niraparib (Zejula) laat goede resultaten zien en schijnt al goedgekeurd te zijn door de EMA.
De vraag is nu: kunnen vrouwen die al eerder behandeld zijn voor eierstok kanker, BRCA-gendraagster zijn, en nog steeds kankervrij, profylactisch behandeld worden met dit middel? Want dat die kanker terugkomt is vrijwel zeker. Maar met dit middel, kan het nog even uitgesteld worden...
Mijn gynaecologe doet er vaag over...
Wie kan hier antwoord op geven?