Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
19 augustus 2017: Update van studieresultaten:
Bron: J Natl Cancer Inst. 2017 Sep 1;109(9). doi: 10.1093/jnci/djx015
Wanneer bij patiënten met in de lever uitgezaaide inoperabele darmkanker vooraf aan systemische chemo met RFA - Radio Frequency Ablation de levertumoren worden weggehaald stijgt de kans op overall overleving op 10 jaar met 25 procent. De leveruitzaaiingen waren bij alle patienten maximaal 10 of minder.
- Na een mediane follow-up van 9.7 jaar, 92 van de 119 (77.3%) patiënten waren overleden: 39 van de 60 (65.0%) in de combinatiegroep van RFA plus systemische chemo en en 53 ovan de 59 (89.8%) patiënten in de groep die alleen systemische chemo kregen.
- Bijna alle patiënten overleden als gevolg van progressie van de ziekte: 35 patiënten in de combinatiegroep, 49 patiënten in de groep die alleen systemische chemo kreeg. Er was een statistisch significant verschil tussen de twee groepen: (hazard ratio = 0.58, 95% confidence interval = 0.38 to 0.88, P = .01).
- 3-jaars overall overleving (OS) was in de combinatiegroep 56.9% (95% CI = 43.3% to 68.5%) versus 55.2% (95% CI = 41.6% to 66.9%) voor de groep die alleen systemische chemo had gekregen.
- 5-jaars overall overleving (OS) was in de combinatiegroep 43.1% (95% CI = 30.3% to 55.3%) versus 30.3% (95% CI = 19.0% to 42.4%) voor de groep die alleen systemische chemo had gekregen.
- 8-jaars overall overleving (OS) 35.9% (95% CI = 23.8% to 48.2%) versus 8.9% (95% CI = 3.3% to 18.1%) voor de groep die alleen systemische chemo had gekregen.
Deze resultaten corresponderen met de resultaten uit de reviewstudie, zie in gerelateerde artikelen, die op ASCO 2017 werd gepubliceerd.
Zie verder hieronder. Het gaat om dezelfde studie maar nu weer wat later geanalyseerd.
25 april 2017: Bron: Journal of the National Cancer Institute
Wanneer bij darmkankerpatiënten met in de lever uitgezaaide darmkanker de levertumoren worden weggehaald via RFA - Radio Frequency Ablation gevolgd door systemische chemo dan hebben zij 42 procent meer kans om na 8 jaar nog te leven in vergelijking met als zij alleen systemische chemo zouden krijgen. Na 8 jaar was het verschil van patienten die nog in leven waren 35.9% vs 8.9%. Dit is volgens de onderzoekers de eerste studie die aantoont dat RFA - Radio Frequency Ablation (en denk ook aan TACE = Transarteriële Chemo Embolisatie behandelingen en LITT = Laser-induced Interstitial Thermotherapy en Nanoknife tegenwoordig) naast systemische chemo bewijst een betere behandeling te zijn dan alleen systemische chemo. Bedenk dat deze studie al startte in 2002 maar werd gesloten in 2007 omdat te weinig patienten zich hadden aangemeld.
From April 2002, patients were recruited from 22 centers in Europe. The trial was prematurely closed for poor accrual because of physician’s preferences in treatment modalities in June 2007, with 60 patients in the experimental arm and 59 patients in the control arm.
Onvoorstelbaar toch dat Nederlandse ziekenhuizen geen patiënten konden vinden voor deze aanpak gezien de grote aantallen patiënten die naar het buitenland gingen voor deze aanpak.
Maar met deze studie gegevens, ook Nederlandse ziekenhuizen deden dus mee aan deze studie en verschillende Nederlandse ondezoekers schreven mee aan dit studierapport (zie auteurgegevens in studierapport), is een aanvraag tot vergoeding van RFA en/of TACE bij bv. dr. Vogl wellicht nu wel te verkrijgen bij uw ziektekostenverzekeraar?
Raadpleeg ook deze lijst van complementaire niet-toxische middelen bij darmkanker die wellicht u nog meer kans geven op overall overleving of op z'n minst met betere kwaliteit van leven.
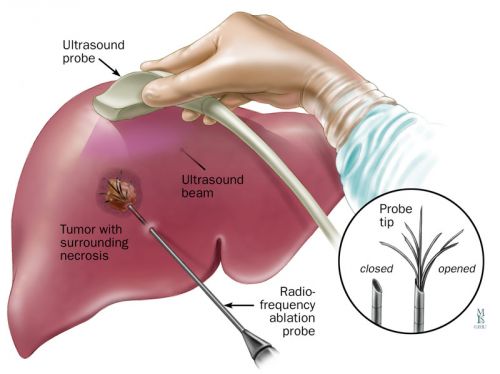
Zo werkt RFA- Radio Frequency Ablation: Tekst gaat onder foto verder:
Studieresultaten:
Van de totaal 119 patienten die meededen aan deze studie waren er 92 (77.3%) na 8 jaar overleden: 39 van de 60 patiënten (65.0%) in de RFA plus chemo groep versus 53 van de 59 patiënten (89.9%) in de groep die alleen systemische chemo kregen (hazard ratio = 0.58, P = .01). De meste patiënten overleden aan de gevolgen van progressie van hun ziekte: 35 van de 39 patiënten uit de RFA plus chemo groep en 49 van de 53 patibnten uit de groep die alleen systemsiche chemo kregen. Overall overleving was 56.9% versus 55.2% na 3 jaar, 43.1% versuss 30.3% na 5 jaar,en 35.9% versuss 8.9% na 8 jaar Mediane overall overleving was 45.6 maanden versus 40.5 maanden respectievelijk.
Conclusie van de onderzoekers was dan ook:: “Deze fase II studie is de eerste gerandomiseerde studie die aantoont dat een "agressieve" benadering van inoperabele in de lever uitgezaaide darmkanker de overall overleving van deze patiëntengroep kan verlengen”
Hier wat grafieken uit het studierapport: Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial dat gratis is in te zien. Onderaan het abstract en referentielijst.
A total of 119 patients were randomly assigned to either systemic treatment alone or combined modality treatment (systemic plus local treatment). Patient and tumor characteristics appeared balanced between both arms (Table 1).
Baseline characteristics
| Patient and tumor characteristics | Local plus systemic treatment (n = 60) | Systemic treatment (n = 59) |
|---|---|---|
| No. (%) | No. (%) | |
| Age, y | ||
| Median (range) | 64 (31–79) | 61 (38–79) |
| Sex | ||
| Male | 37 (61.7) | 42 (71.2) |
| Female | 23 (38.3) | 17 (28.8) |
| WHO performance status | ||
| 0 | 47 (78.3) | 47 (79.7) |
| 1 | 13 (21.7) | 12 (20.3) |
| No. of liver metastases | ||
| 1 | 15 (25.0) | 7 (11.9) |
| 2 | 6 (10.0) | 4 (6.8) |
| 3 | 8 (13.3) | 7 (11.9) |
| 4 | 9 (15.0) | 8 (13.6) |
| 5 | 6 (10.0) | 10 (16.9) |
| 6 | 3 (5.0) | 9 (15.3) |
| 7 | 6 (10.0) | 8 (13.6) |
| 8 | 3 (5.0) | 2 (3.4) |
| 9 | 4 (6.7) | 4 (6.8) |
| Median | 4.0 | 5.0 |
| Synchronicity of liver metastases | ||
| Metachronous metastases | 37 (61.7) | 31 (52.5) |
| Synchronous metastases* | 23 (38.3) | 28 (47.5) |
| Time from surgery for primary cancer to random assignment, d | ||
| Median (range) | 290 (28–1802) | 308 (30–2754) |
| T stage of primary cancer | ||
| pT2 | 9 (15.0) | 4 (6.8) |
| pT3 | 42 (70.0) | 48 (81.4) |
| pT4 | 9 (15.0) | 6 (10.2) |
| Unknown | 0 (0.0) | 1 (1.7) |
| N stage of primary cancer | ||
| pN0 | 17 (28.3) | 21 (35.6) |
| pN1 | 22 (36.7) | 24 (40.7) |
| pN2 | 20 (33.3) | 12 (20.3) |
| Unknown | 1 (1.7) | 2 (3.4) |
| Adjuvant chemotherapy for primary cancer† | ||
| No | 50 (83.3) | 49 (83.1) |
| Yes | 10 (16.7) | 10 (16.9) |
| Prior chemotherapy for metastatic disease† | ||
| No | 51 (85.0) | 51 (86.4) |
| Yes | 9 (15.0) | 8 (13.6) |
| Previous liver surgery for CRC metastases | ||
| No | 51 (85.0) | 49 (83.1) |
| Yes | 9 (15.0) | 10 (16.9) |
| Route of random assignment† | ||
| Before surgery | 46 (76.7) | 44 (74.6) |
| During surgery | 14 (23.3) | 15 (25.4) |
Liver metastases detected within three months after primary cancer diagnosis. CRC = colorectal cancer; WHO = World Health Organization.
Stratification factors.
Local treatment received in the combined treatment arm
| Radiofrequency/surgery | Method | Total (n = 57) | |
|---|---|---|---|
| RFA only (n = 30) | RFA plus resection* (n = 27) | ||
| No. (%) | No. (%) | No. (%) | |
| Means of radiofrequency administration | |||
| At laparotomy | 25 (83.3) | 26 (96.3) | 51 (89.5) |
| Laparascopically | 1 (3.3) | 0 (0.0) | 1 (1.8) |
| Percutaneously | 4 (13.3) | 0 (0.0) | 4 (7.0) |
| No RFA performed | 0 (0.0) | 1 (3.7)* | 1 (1.8) |
| Worst margin for resected† tumors per patient (n = 27), cm | |||
| ≥1 | NA | 10 (37.0) | – |
| <1 | NA | 16 (59.3) | – |
| Residual tumor | NA | 1 (3.7) | – |
| Worst margin for tumors treated by radiofrequency per patient (n = 56), cm | (n = 26) | (n = 56) | |
| ≥1 | 8 (26.7) | 5 (19.2) | 13 (23.2) |
| <1 | 16 (53.3) | 17 (65.4) | 33 (58.9) |
| No margin | 4 (13.3) | 1 (3.8) | 5 (8.9) |
| Unknown | 2 (6.7) | 3 (11.5) | 5 (8.9) |
| Treatment of at least one liver metastasis unsuccessful | |||
| No | 29 (96.7) | 26 (96.3) | 55 (96.5) |
| Yes | 1 (3.3)‡ | 1 (3.7) | 2 (3.5) |
One patient was ineligible; all lesions were resected at baseline, no RFA done. RFA = radiofrequency ablation.
Resection consisted of one segment or wedge resection(s) (n = 16) or resection of two or more liver segments (n = 11).
For this patient, one lesion could not be successfully treated by RFA because of its close proximity to the stomach.
Zoals gezegd: het studierapport: Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial is gratis in te zien.
Hier het abstract en referentielijst:
In this randomized phase II trial, we found that a combination of aggressive local treatment plus systemic treatment, as compared with systemic treatment alone, improved both progression-free survival and overall survival in patients with unresectable colorectal liver metastases. The addition of local treatment using RFA was clinically beneficial and was associated with a statistically significant improvement in overall survival (P = .01).
Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial
Background: Tumor ablation is often employed for unresectable colorectal liver metastases. However, no survival benefit has ever been demonstrated in prospective randomized studies. Here, we investigate the long-term benefits of such an aggressive approach.
Methods: In this randomized phase II trial, 119 patients with unresectable colorectal liver metastases (n < 10 and no extrahepatic disease) received systemic treatment alone or systemic treatment plus aggressive local treatment by radiofrequency ablation ± resection. Previously, we reported that the primary end point (30-month overall survival > 38%) was met. We now report on long-term OS results. All statistical tests were two-sided. The analyses were according to intention to treat.
Results: At a median follow up of 9.7 years, 92 of 119 (77.3%) patients had died: 39 of 60 (65.0%) in the combined modality arm and 53 of 59 (89.8%) in the systemic treatment arm. Almost all patients died of progressive disease (35 patients in the combined modality arm, 49 patients in the systemic treatment arm). There was a statistically significant difference in OS in favor of the combined modality arm (hazard ratio = 0.58, 95% confidence interval = 0.38 to 0.88, P = .01). Three-, five-, and eight-year OS were 56.9% (95% CI = 43.3% to 68.5%), 43.1% (95% CI = 30.3% to 55.3%), 35.9% (95% CI = 23.8% to 48.2%), respectively, in the combined modality arm and 55.2% (95% CI = 41.6% to 66.9%), 30.3% (95% CI = 19.0% to 42.4%), 8.9% (95% CI = 3.3% to 18.1%), respectively, in the systemic treatment arm. Median OS was 45.6 months (95% CI = 30.3 to 67.8 months) in the combined modality arm vs 40.5 months (95% CI = 27.5 to 47.7 months) in the systemic treatment arm.
Conclusions: This phase II trial is the first randomized study demonstrating that aggressive local treatment can prolong OS in patients with unresectable colorectal liver metastases.
References
Gerelateerde artikelen
- Chemo toedienen in buikholte naast systemische chemo geeft verdubbeling van levensverlenging bij patienten met in buikvlies uitgezaaide darmkanker blijkt uit Nederlandse studie. Vervolgstudie staat open voor nieuwe patienten
- Standaard radiotherapie naast chemo vooraf aan operatie bij rectumkanker geeft zelfde overleving op 5 jaar met meer bijwerkingen in vergelijking met alleen selectieve bestraling als chemo te weinig resultaat geeft copy 1
- Lonsurf (Trifluridine-Tipiracil) plus Bevacizumab (Avastin) geeft betere mediane overall overleving en ziekteprogressievrije tijd dan alleen Trifluridine-Tipiracil bij patienten met recidief van uitgezaaide darmkanker
- Vitamine C hoog gedoseerd naast chemo (FOLFOX +/- bevacizumab) geeft in vergelijking met alleen chemo statistisch significant verschil in mediane overall overleving bij inoperabele onbehandelde uitgezaaide darmkanker met RAS mutatie
- FOLFIRI plus cetuximab geeft betere overall overleving dan Avastin - bevacizumab bij darmkanker KRAS wild type.
- Oxaliplatin plus Xeloda - capecitabine naast bestraling voor operatie van endeldarmkanker geeft alleen maar meer bijwerkingen en geen enkel verschil op ziektevrije tijd en overall overleving en is dus zinloos
- S-1 plus oxaliplatine samen met pembrolizumab geeft hoopvolle resultaten bij patiënten met gevorderde maag / darmkanker met 72 procent objectieve respons copy 1
- mFOLFOX6 - fluorouracil, leucovorin en oxaliplatin (met of zonder radiotherapie) vooraf aan operatie geeft geen betere overall overleving voor gevorderde endeldarmkanker dan alleen fluorouracil (5-FU) plus radiotherapie
- Stoppen met oxaliplatin geeft zelfde resultaten maar minder neuropathie in vergelijking met voortzetting na 6 FOLFOX chemokuren plus panitumumab bij patienten met uitgezaaide gevorderde darmkanker
- CAPOX = Xeloda - capecitabine plus oxaliplatin geeft op 3 jaars meting 10 procent betere ziektevrije tijd (83 vs 73 procent) dan FOLFOX = 5-FU + Leucovarin + oxaliplatin bij darmkanker stadium III
- S-1 plus irinotecan plus bevacizumab versus mFOLFOX6 of CapeOX plus bevacizumab voor uitgezaaide darmkanker geeft betere overall overleving en minder bijwerkingen
- RFA naast systemische chemo geeft betere overall overleving 35.9 procent versus 8.9 procent op 8 jaars meting voor in lever uitgezaaide darmkanker
- Darmkankerpatienten jonger dan 50 jaar krijgen veel te vaak systemische chemo zonder effectiviteit op overleving en ziektevrije tijd in vergelijking met darmkankerpatienten ouder dan 50 jaar.
- FOLFOXIRI + Bevacizumab geeft betere progressievrije tijd (9,7 vs 12,1 maanden) dan FOLFIRI + avastin als eerste lijns behandeling bij inoperabele uitgezaaide darmkanker
- Oxaliplatin voegt niets toe aan effectiviteit bij ouderen met darmkanker en bij darmkanker stadium II
- Chemo bij endeldarmkanker heeft weinig effect op overlevingsduur
- Chemo bij darmkanker: Xeloda - Capecitabine bewezen beter alternatief voor 5-FU bij uitgezaaide darmkanker
- Chemo: Hoe langer gewacht met chemo na operatie van darmkanker tumoren, hoe groter de kans op een recidief, blijkt uit grote meta analyse van 10 gerandomiseerde studies met totaal ruim 15.000 darmkankerpatienten
- Chemo na operatie bij darmkankerpatiënten stadium II lijkt zinloos en levert slechts 5% extra overlevingen.op.
- Historisch overzicht van nut van chemo bij darmkanker na operatie, gebaseerd op een aantal verschillende studies.
- Ibuprofen gaat diarree tegen veroorzaakt door gebruik van Xeloda - Capecitabine volgens ervaringen van kankerpatienten.
- Peritoneal Carcinomatosis - uitzaaiingen in de lymfklieren van de buikholte en op de buikwand vanuit dikke darmkanker geeft slechtere prognose op overleving en ziektevrije tijd dan bij uitzaaiingen in andere organen bij intraveneuze chemo
- Xeloda - Capecitabine is in Europa al jaren toegestaan als eerstelijns behandeling bij postoperatieve behandelingen van al of niet uitgezaaide darmkanker.
- Chemo bij darmkanker: een overzicht van artikelen en belangrijke studies




Plaats een reactie ...
Reageer op "RFA naast systemische chemo geeft betere overall overleving 35.9 procent versus 8.9 procent op 8 jaars meting voor in lever uitgezaaide darmkanker"