Aan dit artikel is enkele uren gewerkt. Opzoeken, vertalen, plaatsen enz. Als u ons wilt ondersteunen dan kan dat via een al of niet anonieme donatie. Elk bedrag is welkom hoe klein ook. Klik hier als u ons wilt helpen kanker-actueel online te houden. Wij zijn een ANBI organisatie dus uw donatie is in principe aftrekbaar voor de inkomstenbelasting.
25 april 2017: lees ook dit artikel:
29 mei 2012: Bron: Anticancer Res. 2011 Dec;31(12):4581-7
Trans Arteriële Chemo Embolisatie - TACE met zogeheten 'drug-eluting beads' levert langere ziektevrije tijd en betere kwaliteit van leven op voor patiënten met in de lever uitgezaaide darmkanker. Dit blijkt uit een Italiaanse studie met 108 patiënten met vergevorderde darmkanker stadium III en IV.
De drug-eluting beads is een techniek waarbij via een injectie of katheder met chemo geimpregneerde bolletjes worden ingebracht die kleine hoeveelheden chemo blijven afgeven gedurende een bepaalde periode. Deze methode lijkt op inwendige bestraling maar dan met chemo.
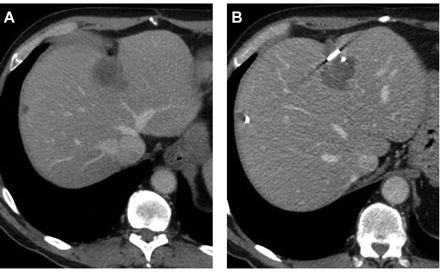
Foto: Scan van een patient voor en na de TACE met de verpakte chemo
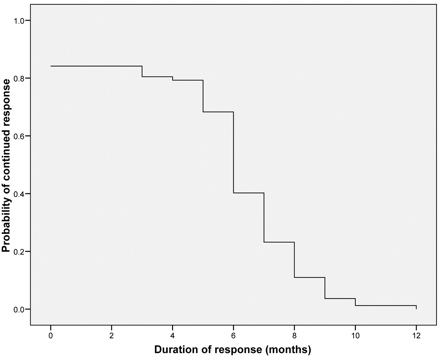
En hier de grafiek van de ziektevrije tijd:
Het volledige studierapport: Trans-Arterial Chemoembolization of Metastatic Colorectal Carcinoma to the Liver Adopting DC Bead®, Drug-eluting Bead Loaded with Irinotecan: Results of a Phase II Clinical Study is gratis in te zien.
Deze animatie laat zien hoe de methode werkt.
In dit filmpje kunt u zien hoe de bolletjes worden klaargemaakt voor gebruik en hoe deze worden ingebracht. Bij primaire leverkanker wordt deze techniek al heel lang gebruikt. Bij andere vormen van kanker waaronder uitgezaaide darmkanker en longkanker wordt deze techniek ook onderzocht met vaak betere resultaten dan met systemische chemo of directe TACE - Trans Arteriële Chemo Embolisatie.
Heir het abstract van de studie. Als u hier klikt kunt u een volledig studie rapport gratis inzien van een tussen evaluatie van deze studie van vorig jaar. Inclusief referentielijst die ook onderaan dit artikel staat vermeld.
Het volledige studierapport van de meest recente presentatie kunt u hier tegen betaling inzien.
TACE with drug-eluting beads loaded with irinotecan could be proposed as palliative therapy for unresectable and chemotherapy resistant liver metastases from colectoral cancer
Trans-arterial chemoembolization of metastatic colorectal carcinoma to the liver adopting DC Bead®, drug-eluting bead loaded with irinotecan: results of a phase II clinical study.
Source
Unit of Oncological Interventional Radiology, IOV (IRCCS), Padova, Italy. camillo.aliberti@ioveneto.it
Abstract
Trans-arterial chemoembolization (TACE) is a promising locoregional therapy for the treatment of primary hepatic tumors and liver metastases. The aim of the study was to define the activity and outcome of using DC Bead, drug-eluting bead, a spherical embolic device capable of being loaded with irinotecan.
PATIENTS AND METHODS:
We conducted a double institutional, single arm, phase II clinical study to evaluate TACE adopting this device in 82 patients presenting with metastatic colorectal carcinoma to the liver after failing chemotherapy. The primary endpoints were tumor shrinkage, safety, feasibility, compliance, and overall survival. RECIST criteria were used to assess responses. Quality of life (QoL) was addressed using Edmonton SAS improvement scale.
RESULTS:
Out of 103 patients considered, 82 were enrolled and underwent a total of 185 treatments of TACE. The median number of TACE was 2.2 (1-4). A post-embolization syndrome was frequently observed. Adverse observed effects were: right upper quadrant pain (40%), fever (80%), nausea (27%) and increased transaminases (70%). The median follow-up was 29 months. Within one month after treatment, each patient received a computed tomograpic scan. It showed reduction of metastatic contrast enhancement in all patients. Responses were 78% at 3 months. After the first treatment, 75 out 82 patients declared an improvement of their well being lasting more than 18 weeks. The median duration of response was 6 (range 3-10) months; the median follow up was 29 (range 7-48) months. The median survival was 25 (range 6-34) months, with progression free survival at 8 (range 4-16) months.
CONCLUSION:
We suggest that TACE adopting DC Bead®, drug-eluting bead loaded with irinotecan could be proposed as palliative therapy for unresectable and chemotherapy resistant liver metastases from CRC.
- PMID:
- 22199334
- [PubMed - indexed for MEDLINE]
-
References
1. Wood CB, Gillis CR, Blumgart LH. A retrospective study of the natural history of patients with liver metastases from colorectal cancer. Clin Oncol. 1976;2:285–288. [PubMed]2. Weitz J, Koch M, Debus J, Hohler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365:153–165. [PubMed]3. Bentrem DJ, Dematteo RP, Blumgart LH. Surgical therapy for metastatic disease to the liver. Annu Rev Med. 2005;56:139–156. [PubMed]4. Adam R, Aloia T, Lévi F, et al. Hepatic resection after rescue cetuximab treatment for colorectal liver metastases previously refractory to conventional systemic therapy. J Clin Oncol. 2007;25:4593–4602. [PubMed]5. Garufi C, Torsello A, Tumulo S, et al. POCHER (preoperative chemotherapy for hepatic resection) study with cetuximab (C-225) plus chronomodulated (chrono) CPT-11/5-fluorouracil (5-FU)/leucovorin (FA)/oxaliplatin (L-OHP) (CPT-FFL) in colorectal liver metastases (CLM) J Clin Oncol. 2009;27(15S):e15020.6. Falcone A, Masi G, Loupakis F, et al. FOLFOXIRI (irinotecan, oxaliplatin and infusional 5FU/LV) in combination with bevacizumab (BV) in the first-line treatment of metastatic colorectal cancer (mCRC): a phase II study by the G.O.N.O. group. J Clin Oncol. 2008;26(15S):4031.7. Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed]8. Kemeny N, Fata F. Hepatic-arterial chemotherapy. Lancet Oncol. 2001;2:418–428. [PubMed]9. Hohn DC, Rayner AA, Economou JS, Ignoffo RJ, Lewis BJ, Stagg RJ. Toxicities and complications of implanted pump hepatic arterial and intravenous floxuridine infusion. Cancer. 1986;57:465–470. [PubMed]10. Mocellin S, Pasquali S, Nitti D. Fluoropyrimidine-HAI (hepatic arterial infusion) versus systemic chemotherapy (SCT) for unresectable liver metastases from colorectal cancer. Cochrane Database Syst Rev. 2009;(3):CD007823. [PubMed]11. Lorenz M, Müller HH. Randomized, multicenter trial of fluorouracil plus leucovorin administered either via hepatic arterial or intravenous infusion versus fluorodeoxyuridine administered via hepatic arterial infusion in patients with nonresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2000;18:243–254. [PubMed]12. Tanaka T, Arai Y, Inaba Y, et al. Radiologic placement of side-hole catheter with tip fixation for hepatic arterial infusion chemotherapy. J Vasc Interv Radiol. 2003;14:63–68. [PubMed]13. Therasse P, Arbuck SG, Eisenhauer EA, et al. European Organization for Research and Treatment of Cancer; National Cancer Institute of the United States; National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors.
J Natl Cancer Inst. 2000;92:205–216. [PubMed]14. de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. [PubMed]15. Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. [PubMed]16. Bouchahda M, Tanaka K, Adam R, et al. Hepatic artery chronomodulated infusion of irinotecan, 5-fluorouracil and oxaliplatin against liver metastases in heavily pretreated patients with colorectal cancer. J Clin Oncol. 2004;22(14S):3722.17. Boige V, Malka D, Elias D, et al. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 in unresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol. 2008;15:219–226. [PubMed]18. Kerr DJ, McArdle CS, Ledermann J, et al. Intrahepatic arterial versus intravenous fluorouracil and folinic acid for colorectal cancer liver metastases: a multicentre randomised trial. Lancet. 2003;361:368–373. [PubMed]19. Kemeny NE, Niedzwiecki D, Hollis DR, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: a randomized trial of efficacy, quality of life, and molecular markers (CALGB 9481) J Clin Oncol. 2006;24:1395–1403. [PubMed]20. Bouchahda M, Adam R, Giacchetti S, et al. Rescue chemotherapy using multidrug chronomodulated hepatic arterial infusion for patients with heavily pretreated metastatic colorectal cancer. Cancer. 2009;115:4990–4999. [PubMed]21. Ensminger WD. Intrahepatic arterial infusion of chemotherapy: pharmacologic principles. Semin Oncol. 2002;29:119–125. [PubMed]22. Allen PJ, Nissan A, Picon AI, et al. Technical complications and durability of hepatic artery infusion pumps for unresectable colorectal liver metastases: an institutional experience of 544 consecutive cases. J Am Coll Surg. 2005;201:57–65. [PubMed]23. Scaife CL, Curley SA, Izzo F, et al. Feasibility of adjuvant hepatic arterial infusion of chemotherapy after radiofrequency ablation with or without resection in patients with hepatic metastases from colorectal cancer. Ann Surg Oncol. 2003;10:348–354. [PubMed]24. Hildebrandt B, Pech M, Nicolaou A, et al. Interventionally implanted port catheter systems for hepatic arterial infusion of chemotherapy in patients with colorectal liver metastases: a Phase II-study and historical comparison with the surgical approach. BMC Cancer. 2007;7:69. [PMC free article] [PubMed]25. Rougier P, Laplanche A, Huguier M, et al. Hepatic arterial infusion of floxuridine in patients with liver metastases from colorectal carcinoma: long-term results of a prospective randomized trial. J Clin Oncol. 1992;10:1112–1118. [PubMed]
Gerelateerde artikelen
- Darmkanker: TACE en LITT (of soms RFA) verlengt leven significant van darmkankerpatienten met uitzaaiingen in de lever
- Darmkanker: Trans Arteriële Chemo Embolisatie - TACE met zogeheten 'drug-eluting beads' levert langere ziektevrije tijd en betere kwaliteit van leven op voor patienten met in de lever uitgezaaide darmkanker
- Darmkanker.:LITT behandeling - Laser-induced Interstitial Thermotherapy - van levertumoren (dr. Vogl) veruit superieur in overlevingstijd aan lokale en regionale chemo bij uitgezaaide darmkanker stadium 4.
- Darmkanker: TACE - Transarteriële Chemo Embolisatie samen met systemische chemo geeft significant langere overlevingstijd voor darmkankerpatiënten met uitzaaiïngen in de lever blijkt uit gerandomiseerde fase II studie
- Darmkanker: overzicht van studies met TACE - Trans Arteriele Chemo Embolisatie voor levertumoren ontstaan vanuit vormen van darmkanker




Plaats een reactie ...
Reageer op "Darmkanker: Trans Arteriële Chemo Embolisatie - TACE met zogeheten 'drug-eluting beads' levert langere ziektevrije tijd en betere kwaliteit van leven op voor patienten met in de lever uitgezaaide darmkanker"