19 juni 2019: lees ook dit artikel:
11 september 2017: Bron: ESMO 2017
Plaats van de primaire tumor en niet de KRAS mutatie status is bepalend voor therapeutische effect van SIRT - Yttrium-90 naast chemotherapie voor in lever uitgezaaide darmkanker.
Wanneer darmkankerpatienten met in de lever uitgezaaide inoperabele levertumoren naast chemotherapie (FOLFOX + bevacizumab / Avastin) SIRT = inwendige bestraling met Yttrium-90 krijgen heeft dat in algemene zin geen statistisch significant effect op de progressievrije tijd (PFS) en mediane overall overleving (OS). Echter uit een analyse van verschillend samengestelde subgroepen blijkt er tussen de groepen patiënten waarbij de primaire darmtumor rechtszijdig was wel een aanmerkelijk verschil in overall overleving te zijn. Namelijk een mediane overall overleving van 22,0 maanden voor de SIRT groep versus 17,1 maanden voor de chemogroep zonder SIRT.
Dit blijkt uit een analyse van drie studies uitgevoerd door Harpreet Wasan, head of the gastrointestinal clinical research programme at Hammersmith Hospital, Imperial College London, UK and Australian colleagues. Zij analyseerden drie studies met een min of meer gelijkwaardige patientepopulatie en studiedoel: de studies FOXFIRE, ISRCTN83867919, SIRFLOX NCT00724503, en FOXFIRE-Global NCT01721954).
TEskt gaat onder grafiek verder
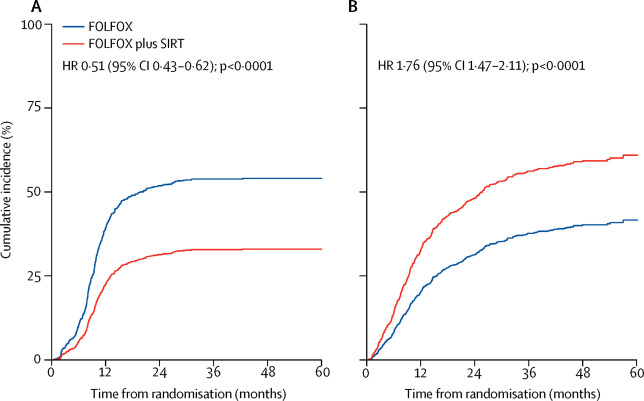
De studies hebben alle een eerstelijns behandeling met SIRT - Yttrium-90 geëvalueerd plus chemotherapie met FOLFOX (+/- bevacizumab) bij 1.103 patiënten met uitgezaaide darmkanker en niet operabele leveruitzaaiingen, die eerder nog geen chemo hadden gehad
De patiënten werden gerandomiseerd ingedeeld in een groep die standaard chemo met oxaliplatin kregen met of zonder bevacizumab - Avastin (controle groep, n = 549) of een groep met dezelfde chemotherapie plus SIRT (Yttrium-90 = inwendige bestraling via de bloedbaan van de lever) (n = 554). Patiënten met ascites, levercirrose of portalhypertensie werden uitgesloten van de analyse.
Het primaire eindpunt van alle studies was overall overleving (OS), en in deze bijgewerkte analyse werd de invloed geanalyserd van een KRAS mutatie en aan welke kant van de darm de oorsprong van de primaire tumor was.
Uit de analyse kwamen de volgende resultaten en werden op ESMO 2017 geporesenteerd gisteren. Ik vertaal deze maar niet want zijn ook in het Engels helder en duidelijik lijkt mij.
Een speciaal commentaar op het studierapport: Wasan HS, et al. First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials. Lancet Oncol. 2017 Aug 3. is ook in The Lancet gepubliceerd.
Hier het abstract vna de studie
This analysis adds to the increasing literature for primary tumour location-based differences in metastatic colorectal cancer mCRC outcomes with treatments, and may support a side-based approach to patient selection for SIRT. However, SIRT in the first-line setting cannot be recommended today.
First-line selective internal radiotherapy plus chemotherapy versus chemotherapy alone in patients with liver metastases from colorectal cancer (FOXFIRE, SIRFLOX, and FOXFIRE-Global): a combined analysis of three multicentre, randomised, phase 3 trials
Summary
Background
Data suggest selective internal radiotherapy (SIRT) in third-line or subsequent therapy for metastatic colorectal cancer has clinical benefit in patients with colorectal liver metastases with liver-dominant disease after chemotherapy. The FOXFIRE, SIRFLOX, and FOXFIRE-Global randomised studies evaluated the efficacy of combining first-line chemotherapy with SIRT using yttrium-90 resin microspheres in patients with metastatic colorectal cancer with liver metastases. The studies were designed for combined analysis of overall survival.
Methods
FOXFIRE, SIRFLOX, and FOXFIRE-Global were randomised, phase 3 trials done in hospitals and specialist liver centres in 14 countries worldwide (Australia, Belgium, France, Germany, Israel, Italy, New Zealand, Portugal, South Korea, Singapore, Spain, Taiwan, the UK, and the USA). Chemotherapy-naive patients with metastatic colorectal cancer (WHO performance status 0 or 1) with liver metastases not suitable for curative resection or ablation were randomly assigned (1:1) to either oxaliplatin-based chemotherapy (FOLFOX: leucovorin, fluorouracil, and oxaliplatin) or FOLFOX plus single treatment SIRT concurrent with cycle 1 or 2 of chemotherapy. In FOXFIRE, FOLFOX chemotherapy was OxMdG (oxaliplatin modified de Gramont chemotherapy; 85 mg/m2 oxaliplatin infusion over 2 h, L-leucovorin 175 mg or D,L-leucovorin 350 mg infusion over 2 h, and 400 mg/m2 bolus fluorouracil followed by a 2400 mg/m2 continuous fluorouracil infusion over 46 h). In SIRFLOX and FOXFIRE-Global, FOLFOX chemotherapy was modified FOLFOX6 (85 mg/m2 oxaliplatin infusion over 2 h, 200 mg leucovorin, and 400 mg/m2 bolus fluorouracil followed by a 2400 mg/m2 continuous fluorouracil infusion over 46 h). Randomisation was done by central minimisation with four factors: presence of extrahepatic metastases, tumour involvement of the liver, planned use of a biological agent, and investigational centre. Participants and investigators were not masked to treatment. The primary endpoint was overall survival, analysed in the intention-to-treat population, using a two-stage meta-analysis of pooled individual patient data. All three trials have completed 2 years of follow-up. FOXFIRE is registered with the ISRCTN registry, number ISRCTN83867919. SIRFLOX and FOXFIRE-Global are registered with ClinicalTrials.gov, numbers NCT00724503 (SIRFLOX) and NCT01721954 (FOXFIRE-Global).
Findings
Between Oct 11, 2006, and Dec 23, 2014, 549 patients were randomly assigned to FOLFOX alone and 554 patients were assigned FOLFOX plus SIRT. Median follow-up was 43·3 months (IQR 31·6–58·4). There were 411 (75%) deaths in 549 patients in the FOLFOX alone group and 433 (78%) deaths in 554 patients in the FOLFOX plus SIRT group. There was no difference in overall survival (hazard ratio 1·04, 95% CI 0·90–1·19; p=0·61). The median survival time in the FOLFOX plus SIRT group was 22·6 months (95% CI 21·0–24·5) compared with 23·3 months (21·8–24·7) in the FOLFOX alone group. In the safety population containing patients who received at least one dose of study treatment, as treated, the most common grade 3–4 adverse event was neutropenia (137 [24%] of 571 patients receiving FOLFOX alone vs 186 (37%) of 507 patients receiving FOLFOX plus SIRT). Serious adverse events of any grade occurred in 244 (43%) of 571 patients receiving FOLFOX alone and 274 (54%) of 507 patients receiving FOLFOX plus SIRT. 10 patients in the FOLFOX plus SIRT group and 11 patients in the FOLFOX alone group died due to an adverse event; eight treatment-related deaths occurred in the FOLFOX plus SIRT group and three treatment-related deaths occurred in the FOLFOX alone group.
Interpretation
Addition of SIRT to first-line FOLFOX chemotherapy for patients with liver-only and liver-dominant metastatic colorectal cancer did not improve overall survival compared with that for FOLFOX alone. Therefore, early use of SIRT in combination with chemotherapy in unselected patients with metastatic colorectal cancer cannot be recommended. To further define the role of SIRT in metastatic colorectal cancer, careful patient selection and studies investigating the role of SIRT as consolidation therapy after chemotherapy are needed.
Funding
Bobby Moore Fund of Cancer Research UK, Sirtex Medical.
Gerelateerde artikelen
- Yttrium-90 radio-embolisatie van levertumoren gevolgd door immuuntheapie met Durvalumab en Tremelimumab bij darmkankerpatiënten met microsatelliet stabiel is veilig toe te passen maar immuuntherapie slaat niet aan daardoor
- SIRT - inwendige bestraling (Yttrium-90) naast chemotherapie geeft alleen bij rechtszijdige darmtumoren een verschil in overall overleving OS = 22.0 vs 17.1 maanden bij uitgezaaide darmkanker
- SIRT - Yttrium-90 - een vorm van inwendige bestraling via zogeheten microsferen officieel opgenomen in de richtlijnen voor behandelen van levertumoren
- Bestraling - Radio embolisatie met nieuwe techniek Yttrium-90 voor levertumoren nu ook in Nederland beschikbaar in studieverband. Artikel geplaatst 20 december 2009



 FOXFIRE trial investigators†
FOXFIRE trial investigators†
Plaats een reactie ...
Reageer op "SIRT - inwendige bestraling (Yttrium-90) naast chemotherapie geeft alleen bij rechtszijdige darmtumoren een verschil in overall overleving OS = 22.0 vs 17.1 maanden bij uitgezaaide darmkanker"