BiTE = Bispecifieke T-cel-engager medicijnen en behandelingen blijkt een hoopvolle nieuwe variant van immuuntherapie met gemoduleerde CAR-T cellen bij de behandeling van kanker. Ook bij vormen van kanker met solide tumoren.
Bi-specifieke antilichamen (BiTE) zijn opgebouwd uit twee verschillende antilichamen waardoor ze twee verschillende soorten antigenen kunnen herkennen om een immuunrespons in het lichaam op gang te brengen. Dat betekent dat bi-specifieke antilichamen het afweersysteem van de patiënt activeren om de ziekte te bestrijden.
In een reviewstudie wordt een overzicht gegeven van de ontwikkelingen op dit gebied (abstract staat verderop in dit artikel).
Hier een stuk tekst uit de introductie van de studie vertaald:
Op T-cellen gebaseerde immuuntherapieën bij kanker hebben de klinische praktijk van kankerbehandeling getransformeerd door T-cellen te targeten en te mobiliseren om kwaadaardige tumorcellen te vernietigen.
Afhankelijk van de werkingsmechanismen kunnen op T-cellen gebaseerde vormen van immuuntherapieën hoofdzakelijk in twee klassen worden verdeeld: de ene tegen immuunsuppressieve factoren, vertegenwoordigd door Anti-PD medicijnen - checkpointremmers (ICI's).
De andere vorm is gericht op immuunstimulerende routes, vertegenwoordigd door chimere antigeenreceptor (CAR) T. cellen en op T-cel-aangrijpende bispecifieke antilichamen (bsAbs). [1,2,3]
Anti-PD medicijnen - checkpointremmers (ICI's) hebben een revolutie teweeggebracht in de behandeling van kanker in de kliniek, vooral bij verschillende gevorderde solide tumoren, bijvoorbeeld melanoom en niet-kleincellige longkanker. [4,5,6,7]
Ze belemmeren de immuunontsnapping van de tumor door belangrijke immunosuppressieve moleculen zoals geprogrammeerde celdood 1 (PD-1) en zijn ligand (PD-L1) te blokkeren en de ‘rem’ van cytotoxische T-cellen vrij te geven om tumorcellen te elimineren. Maar de werking van Anti-PD medicijnen - checkpointremmers (ICI's) blijven beperkt. [8]
Een belangrijke reden hiervoor is het ontbreken van een voldoende aantal tumor-infiltrerende immuuncellen (TIL's), voornamelijk T-cellen, op de tumorplaats, die een koud fenotype wordt genoemd [9].
CAR T-celtherapie is een nieuw ontwikkelde adoptieve celtherapie door T-cellen genetisch te manipuleren om een CAR tot expressie te brengen die bestaat uit intracellulaire T-cel-signaleringsdomeinen en een extracellulaire antigeenherkenningsstructuur die zich richt op tumor-geassocieerde antigenen (TAA's), waardoor T-cellen worden omgeleid en geactiveerd naar kwaadaardige cellen specifiek uitroeien. [10]
De bereiding van CAR T-cellen omvat voornamelijk isolatie van T-cellen bij patiënten, genetische modificatie van T-cellen, expansie van T-cellen in vitro en infusie van bewerkte T-cellen bij patiënten, wat echter een complex en tijdrovend proces is [11].
De andere alternatieve benadering om T-cellen om te leiden naar doelcellen is T-cel-aangrijpende bsAbs met unieke functie-aansprekende TAA's op kankercellen en celoppervlaktemoleculen op T-cellen. Bispecifieke T-cel-engager (BiTE) onderscheidt zich als een nieuwe subklasse van T-cel-aangrijpende bsAbs met veelbelovende klinische resultaten bij de behandeling van kanker. En de vergelijking van deze drie op T-cellen gebaseerde immuuntherapieën is samengevat in Tabel 1.
Een afbeelding uit het studieverslag dat het werkings mechanisme van een Bi-specifieke antilichamen (BiTE) verbeeldt:
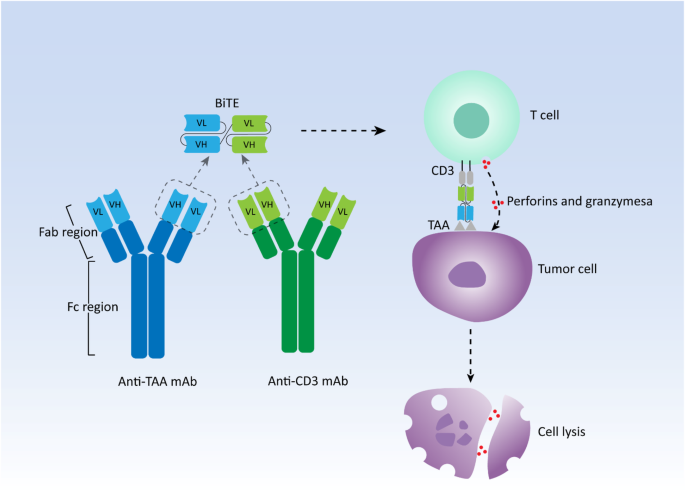
The schematic representation of structure and mechanism of action of canonical bispecific T-cell engager (BiTE). mAb: Monoclonal antibody; VH: Heavy chain variable region; VL: Light chain variable region; TAA: Tumor-associated antigen
De onderzoekers concluderen:
Conclusies:
Naarmate het landschap van T-cellen en hun rol in de immuniteit tegen kanker evolueert, heeft de ontwikkeling van op T-cellen gebaseerde immuuntherapieën het afgelopen decennium een enorme doorbraak bereikt. In het bijzonder hebben de BiTE-antilichamen die T-cellen omleiden om tumorcellen te doden, gunstige klinische resultaten vertoond bij R/R-hematopoëtische maligniteiten, evenals bij solide tumoren met voorlopig bewijs van klinische voordelen.
Er blijft echter een aanzienlijk aantal patiënten die resistent zijn tegen de BiTE-therapie en geen duurzame respons kunnen bereiken.
Huidig onderzoek heeft aangetoond dat antigeenverlies en immunosuppressieve factoren, vooral de opregulatie van remmende checkpointmoleculen, de twee belangrijkste mechanismen zijn, die verantwoordelijk zijn voor het mislukken van de behandeling.
Daarom worden momenteel strategieën met betrekking tot het verbeteren van BiTE-constructies en het ontwikkelen van nieuwe T-cel-engager-antilichamen met hogere antigeen-aviditeiten en meerdere doelwitten onderzocht in een reeks preklinische en klinische onderzoeken, evenals combinatietherapieën met BiTE-antilichamen en andere therapeutische benaderingen.
Naast T-cellen krijgen aangeboren cel- of aangeboren-achtige cel-engagers die zich richten op aangeboren immuniteit steeds meer aandacht en hebben ze krachtige antitumoractiviteit getoond bij verschillende vormen van kanker, wat een oriëntatie op de toekomst weerspiegelt.
Voor het volledige studierapport dat heel uitgebreid ingaat op de verschillende vormen van T-cel-aangrijpende bispecifieke antilichamen (bsAbs) en ook de lopende studies noemt en studies in combinatie met chemotherapie of andere reguliere behandelingen is gratis in te zien.
Klik op de titel van het abstract:
- Review
- Open access
- Published:
The landscape of bispecific T cell engager in cancer treatment
Biomarker Research volume 9, Article number: 38 (2021)
Abstract
T cell-based immunotherapies have revolutionized treatment paradigms in various cancers, however, limited response rates secondary to lack of significant T-cell infiltration in the tumor site remain a major problem. To address this limitation, strategies for redirecting T cells to treat cancer are being intensively investigated, while the bispecific T cell engager (BiTE) therapy constitutes one of the most promising therapeutic approaches. BiTE is a bispecific antibody construct with a unique function, simultaneously binding an antigen on tumor cells and a surface molecule on T cells to induce tumor lysis. BiTE therapy represented by blinatumomab has achieved impressive efficacy in the treatment of B cell malignancies. However, major mechanisms of resistance to BiTE therapy are associated with antigen loss and immunosuppressive factors such as the upregulation of immune checkpoints. Thus, modification of antibody constructs and searching for combination strategies designed to further enhance treatment efficacy as well as reduce toxicity has become an urgent issue, especially for solid tumors in which response to BiTE therapy is always poor. In particular, immunotherapies focusing on innate immunity have attracted increasing interest and have shown promising anti-tumor activity by engaging innate cells or innate-like cells, which can be used alone or complement current therapies. In this review, we depict the landscape of BiTE therapy, including clinical advances with potential response predictors, challenges of treatment toxicity and resistance, and developments of novel immune cell-based engager therapy.
Availability of data and materials
Not applicable.
References
-
Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. Coinhibitory pathways in immunotherapy for cancer. Annu Rev Immunol. 2016;34:539–73.
-
June CH, Connor RSO, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–5.
-
Goebeler M, Bargou RC. T cell-engaging therapies — BiTEs and beyond. Nat Rev Clin Oncol. 2020;17:418–34.
-
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. New Engl J Med. 2015;373:1627–39.
-
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. New Engl J Med. 2015;373:123–35.
-
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44.
-
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. New Engl J Med. 2015;372:2521–32.
-
Friedrich M, Jasinski-Bergner S, Lazaridou M, Subbarayan K, Massa C, Tretbar S, et al. Tumor-induced escape mechanisms and their association with resistance to checkpoint inhibitor therapy. Cancer Immunol Immunother. 2019;68:1689–700.
-
Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-γ and IL-12. Immunity. 2018;49:1148–1161.e7.
-
Singh AK, McGuirk JP. CAR T cells: continuation in a revolution of immunotherapy. Lancet Oncol. 2020;21:e168–78.
-
Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book. 2019;39:433–44.
-
Suurs FV, Lub-de Hooge MN, de Vries EGE, de Groot DJA. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol Therapeut. 2019;201:103–19.
-
Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–66.
-
Thakur A, Huang M, Lum LG. Bispecific antibody based therapeutics: strengths and challenges. Blood Rev. 2018;32:339–47.
-
Wang TT, Ravetch JV. Functional diversification of IgGs through Fc glycosylation. J Clin Invest. 2019;129:3492–8.
-
Löffler A, Kufer P, Lutterbüse R, Zettl F, Daniel PT, Schwenkenbecher JM, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–103.
-
DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482:405–9.
-
Alspach E, Lussier DM, Miceli AP, Kizhvatov I, DuPage M, Luoma AM, et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574:696–701.
-
Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581:100–5.
-
Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619.
-
Klinger M, Benjamin J, Kischel R, Stienen S, Zugmaier G. Harnessing T cells to fight cancer with BiTE® antibody constructs - past developments and future directions. Immunol Rev. 2016;270:193–208.
-
Offner S, Hofmeister R, Romaniuk A, Kufer P, Baeuerle PA. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol. 2006;43:763–71.
-
Dreier T, Lorenczewski G, Brandl C, Hoffmann P, Syring U, Hanakam F, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002;100:690–7.
-
Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, et al. The immunological synapse. Annu Rev Immunol. 2001;19:375–96.
-
Wolf E, Hofmeister R, Kufer P, Schlereth B, Baeuerle PA. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discov Today. 2005;10:1237–44.
-
Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1:36.
-
Engel P, Zhou L, Ord DC, Sato S, Koller B, Tedder TF. Abnormal B lymphocyte delevopment, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39–50.
-
Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blinatumomab: a historical perspective. Pharmacol Therapeut. 2012;136:334–42.
-
Hoelzer D, Bassan R, Dombret H, Fielding A, Ribera JM, Buske C. Acute lymphoblastic leukaemia in adult patients: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v69–82.
-
Einsele H, Borghaei H, Orlowski RZ, Subklewe M, Roboz GJ, Zugmaier G, et al. The BiTE (bispecific T-cell engager) platform: development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer-Am Cancer Soc. 2020;126:3192–201.
-
Liu D, Zhao J, Song Y, Luo X, Yang T. Clinical trial update on bispecific antibodies, antibody-drug conjugates, and antibody-containing regimens for acute lymphoblastic leukemia. J Hematol Oncol. 2019;12:15.
-
Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–8.
-
Topp MS, Gökbuget N, Zugmaier G, Degenhard E, Goebeler M, Klinger M, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120:5185–7.
-
Gökbuget N, Zugmaier G, Klinger M, Kufer P, Stelljes M, Viardot A, et al. Long-term relapse-free survival in a phase 2 study of blinatumomab for the treatment of patients with minimal residual disease in B-lineage acute lymphoblastic leukemia. Haematologica. 2017;102:e132–5.
-
Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131:1522–31.
-
Gökbuget N, Zugmaier G, Dombret H, Stein A, Bonifacio M, Graux C, et al. Curative outcomes following blinatumomab in adults with minimal residual disease B-cell precursor acute lymphoblastic leukemia. Leuk Lymphoma. 2020;61:2665–73.
-
Kiyoi H, Morris JD, Oh I, Maeda Y, Minami H, Miyamoto T, et al. Phase 1b/2 study of blinatumomab in Japanese adults with relapsed/refractory acute lymphoblastic leukemia. Cancer Sci. 2020;111:1314–23.
-
Topp MS, Gökbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, et al. Phase II trial of the anti-CD19 bispecific T cell–engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32:4134–40.
-
Topp MS, Gökbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66.
-
Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera J, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. New Engl J Med. 2017;376:836–47.
-
Locatelli F, Zugmaier G, Mergen N, Bader P, Jeha S, Schlegel P, et al. Blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia: results of the RIALTO trial, an expanded access study. Blood Cancer J. 2020;10:1–5.
-
von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34:4381–9.
-
Martinelli G, Boissel N, Chevallier P, Ottmann O, Gökbuget N, Topp MS, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome–positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35:1795–802.
-
Goebeler M, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol. 2016;34:1104–11.
-
Dufner V, Sayehli CM, Chatterjee M, Hummel HD, Gelbrich G, Bargou RC, et al. Long-term outcome of patients with relapsed/refractory B-cell non-Hodgkin lymphoma treated with blinatumomab. Blood Adv. 2019;3:2491–8.
-
Coyle L, Morley NJ, Rambaldi A, Mason KD, Verhoef G, Furness CL, et al. Open-label, phase 2 study of blinatumomab as second salvage therapy in adults with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Leuk Lymphoma. 2020;61:2103–12.
-
Viardot A, Goebeler M, Hess G, Neumann S, Pfreundschuh M, Adrian N, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127:1410–6.
-
Malik H, Buelow B, Rangaswamy U, Balasubramani A, Boudreau A, Dang K, et al. TNB-486, a novel fully human bispecific CD19 x CD3 antibody that kills CD19-positive tumor cells with minimal cytokine secretion. Blood. 2019;134:4070.
-
Izhak L, Cullen DE, Elgawly M, Luistro L, Johnson S, Bald J, et al. Abstract 3636: potent antitumor activity of duvortuxizumab, a CD19 x CD3 DART® molecule, in lymphoma models. Cancer Res. 2017;77:3636.
-
Reusch U, Duell J, Ellwanger K, Herbrecht C, Knackmuss SH, Fucek I, et al. A tetravalent bispecific TandAb (CD19/CD3), AFM11, efficiently recruits T cells for the potent lysis of CD19(+) tumor cells. Mabs-Austin. 2015;7:584–604.
-
Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–66.
-
Ravandi F, Walter RB, Subklewe M, Buecklein V, Jongen-Lavrencic M, Paschka P, et al. Updated results from phase I dose-escalation study of AMG 330, a bispecific T-cell engager molecule, in patients with relapsed/refractory acute myeloid leukemia (R/R AML). J Clin Oncol. 2020;38:7508.
-
Subklewe M, Stein A, Walter RB, Bhatia R, Wei AH, Ritchie D, et al. Preliminary results from a phase 1 first-in-human study of AMG 673, a novel half-life extended (HLE) anti-CD33/CD3 BiTE® (bispecific T-cell engager) in patients with relapsed/refractory (R/R) acute myeloid leukemia (AML). Blood. 2019;134:833.
-
Nair-Gupta P, Diem M, Reeves D, Wang W, Schulingkamp R, Sproesser K, et al. A novel C2 domain binding CD33xCD3 bispecific antibody with potent T-cell redirection activity against acute myeloid leukemia. Blood Adv. 2020;4:906–19.
-
Anti-CD33/CD3 bispecific antibody GEM 333. Available at: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/anti-cd33-cd3-bispecific-antibody-gem-333. Accessed 20 Dec 2020.
-
Westervelt P, Cortes JE, Altman JK, Long M, Oehler VG, Gojo I, et al. Phase 1 first-in-human trial of AMV564, a bivalent bispecific (2:2) CD33/CD3 T-cell engager, in patients with relapsed/refractory acute myeloid leukemia (AML). Blood. 2019;134:834.
-
Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res. 2013;19:2048–60.
-
Topp MS, Duell J, Zugmaier G, Attal M, Moreau P, Langer C, et al. Anti-B-cell maturation antigen BiTE molecule AMG 420 induces responses in multiple myeloma. J Clin Oncol. 2020;38:775–83.
-
Harrison SJ, Minnema MC, Lee HC, Spencer A, Kapoor P, Madduri D, et al. A phase 1 first in human (FIH) study of AMG 701, an anti-B-cell maturation antigen (BCMA) half-life extended (HLE) BiTE® (bispecific T-cell engager) molecule, in relapsed/refractory (RR) multiple myeloma (MM). Blood. 2020;136:28–9.
-
Pillarisetti K, Powers G, Luistro L, Babich A, Baldwin E, Li Y, et al. Teclistamab is an active T cell–redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Adv. 2020;4:4538–49.
-
Madduri D, Rosko A, Brayer J, Zonder J, Bensinger WI, Li J, et al. REGN5458, a BCMA x CD3 bispecific monoclonal antibody, induces deep and durable responses in patients with relapsed/refractory multiple myeloma (RRMM). Blood. 2020;136:41–2.
-
Costa LJ, Wong SW, Bermúdez A, de la Rubia J, Mateos M, Ocio EM, et al. First clinical study of the B-cell maturation antigen (BCMA) 2+1 T cell engager (TCE) CC-93269 in patients (Pts) with relapsed/refractory multiple myeloma (RRMM): interim results of a phase 1 multicenter trial. Blood. 2019;134:143.
-
Raje NS, Jakubowiak A, Gasparetto C, Cornell RF, Krupka HI, Navarro D, et al. Safety, clinical activity, pharmacokinetics, and pharmacodynamics from a phase I study of PF-06863135, a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM). Blood. 2019;134:1869.
-
Rodriguez C, D'Souza A, Shah N, Voorhees PM, Buelow B, Vij R, et al. Initial results of a phase I study of TNB-383B, a BCMA x CD3 bispecific T-cell redirecting antibody, in relapsed/refractory multiple myeloma. Blood. 2020;136:43–4.
-
Hutchings M, Mous R, Clausen MR, Johnson P, Linton KM, Chamuleau MED, et al. Subcutaneous epcoritamab induces complete responses with an encouraging safety profile across relapsed/refractory B-cell non-Hodgkin lymphoma subtypes, including patients with prior CAR-T therapy: updated dose escalation data. Blood. 2020;136:45–6.
-
Bannerji R, Allan JN, Arnason JE, Brown JR, Advani R, Ansell SM, et al. Odronextamab (REGN1979), a human CD20 x CD3 bispecific antibody, induces durable, complete responses in patients with highly refractory B-cell non-Hodgkin lymphoma, including patients refractory to CAR T therapy. Blood. 2020;136:42–3.
-
Assouline SE, Kim WS, Sehn LH, Schuster SJ, Cheah CY, Nastoupil LJ, et al. Mosunetuzumab shows promising efficacy in patients with multiply relapsed follicular lymphoma: updated clinical experience from a phase I dose-escalation trial. Blood. 2020;136:42–4.
-
Patel K, Michot J, Chanan-Khan AA, Salles GA, Cartron G, Peyrade F, et al. Preliminary safety and anti-tumor activity of XmAb13676, an anti-CD20 x anti-CD3 bispecific antibody, in patients with relapsed/refractory non-Hodgkin's lymphoma and chronic lymphocytic leukemia. Blood. 2019;134:4079.
-
Hutchings M, Carlo-Stella C, Bachy E, Offner FC, Morschhauser F, Crump M, et al. Glofitamab step-up dosing induces high response rates in patients with hard-to-treat refractory or relapsed non-Hodgkin lymphoma. Blood. 2020;136:46–8.
-
IGM Biosciences Presents First Clinical Data from IGM-2323 in Non-Hodgkin’s Lymphoma at 2020 ASH Annual Meeting. Available at: https://igmbio.com/2020/12/05/igm-biosciences-presents-first-clinical-data-from-igm-2323-in-non-hodgkins-lymphoma-at-2020-ash-annual-meeting/. Accessed 15 Mar 2021.
-
Garfall AL, Usmani SZ, Mateos M, Nahi H, van de Donk NWCJ, San-Miguel JF, et al. Updated phase 1 results of teclistamab, a B-cell maturation antigen (BCMA) x CD3 bispecific antibody, in relapsed and/or refractory multiple myeloma (RRMM). Blood. 2020;136:27.
-
Pfizer Reports Positive Clinical Data for BCMA-CD3 Bispecific Antibody (PF-06863135) in Multiple Myeloma. Available at: https://stockhouse.com/news/press-releases/2020/12/07/pfizer-reports-positive-clinical-data-for-bcma-cd3-bispecific-antibody-pf. Accessed 16 Mar 2021.
-
Cohen AD, Harrison SJ, Krishnan A, Fonseca R, Forsberg PA, Spencer A, et al. Initial clinical activity and safety of BFCR4350A, a FcRH5/CD3 T-cell-engaging bispecific antibody, in relapsed/refractory multiple myeloma. Blood. 2020;136:42–3.
-
Chari A, Berdeja JG, Oriol A, van de Donk NWCJ, Rodriguez P, Askari E, et al. A phase 1, first-in-human study of talquetamab, a G protein-coupled receptor family C group 5 member D (GPRC5D) x CD3 bispecific antibody, in patients with relapsed and/or refractory multiple myeloma (RRMM). Blood. 2020;136:40–1.
-
Aldoss I, Uy GL, Vey N, Emadi A, Sayre PH, Walter RB, et al. Flotetuzumab as salvage therapy for primary induction failure and early relapse acute myeloid leukemia. Blood. 2020;136:16–8.
-
Mhawech-Fauceglia P, Zhang S, Terracciano L, Sauter G, Chadhuri A, Herrmann FR, et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology. 2007;50:472–83.
-
Hummel H, Kufer P, Grüllich C, Deschler-Baier B, Chatterjee M, Goebeler M, et al. Phase I study of pasotuxizumab (AMG 212/BAY 2010112), a PSMA-targeting BiTE (bispecific T-cell engager) immune therapy for metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2020;38:124.
-
Tran B, Horvath L, Dorff T, Rettig M, Lolkema MP, Machiels J, et al. Results from a phase I study of AMG 160, a half-life extended (HLE), PSMA-targeted, bispecific T-cell engager (BiTE®) immune therapy for metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. 2020;31(suppl_4):S507–49.
-
Bendell JC, Fong L, Stein MN, Beer TM, Ross A, Gao X, et al. First-in-human phase I study of HPN424, a tri-specific half-life extended PSMA-targeting T-cell engager in patients with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2020;38:5552.
-
Rosenthal MA, Balana C, van Linde ME, Sayehli C, Fiedler WM, Wermke M, et al. ATIM-49 (LTBK-01). AMG 596, AMG 596, a novel anti-EGFRvIII bispecific T cell engager (BITE®) molecule for the treatment of glioblastoma (GBM): planned interim analysis in recurrent GBM (RGBM). Neuro-Oncology. 2019;21:vi283.
-
Amgen. Amgen Presents First Clinical Data for AMG 757 at SITC 2020. https://www.amgen.com/newsroom/press-releases/2020/11/amgen-presents-first-clinical-data-for-amg-757-at-sitc-2020. Accessed 12 Dec 2020.
-
Chao J, Buxó E, Cervantes A, Dayyani F, Lima CMSP, Greil R, et al. Trial in progress: a phase I study of AMG 199, a half-life extended bispecific T-cell engager (HLE BiTE) immune therapy, targeting MUC17 in patients with gastric and gastroesophageal junction (G/GEJ) cancer. J Clin Oncol. 2020;38:TPS4649.
-
Bailis JM, Lutterbuese P, Thomas O, Locher K, Harrold J, Boyle M, et al. Abstract 3364: preclinical evaluation of BiTE®immune therapy targeting MUC17 or CLDN18.2 for gastric cancer. Cancer Res. 2020;80:3364.
-
Piha-Paul S, Starodub A, Karim R, Shafique M, Suarez GT, Ruegg C, et al. 372 single-agent anti-tumor activity in relapsed/refractory solid tumors: interim data from the phase 1 solid tumor trial of AMV564, a novel T-cell engager. J Immunother Cancer. 2020;8:A397.
-
Tabernero J, Melero I, Ros W, Argiles G, Marabelle A, Rodriguez-Ruiz ME, et al. Phase Ia and Ib studies of the novel carcinoembryonic antigen (CEA) T-cell bispecific (CEA CD3 TCB) antibody as a single agent and in combination with atezolizumab: preliminary efficacy and safety in patients with metastatic colorectal cancer (mCRC). J Clin Oncol. 2017;35:3002.
-
Yu S, Zhang J, Yan Y, Yao X, Fang L, Xiong H, et al. A novel asymmetrical anti-HER2/CD3 bispecific antibody exhibits potent cytotoxicity for HER2-positive tumor cells. J Exp Clin Cancer Res. 2019;38:355.
-
Song L, Xue J, Zhang J, Li S, Liu D, Zhou T. Mechanistic prediction of first-in-human dose for bispecific CD3/EpCAM T-cell engager antibody M701, using an integrated PK/PD modeling method. Eur J Pharm Sci. 2021;158:105584.
-
Ishiguro T, Sano Y, Komatsu S, Kamata-Sakurai M, Kaneko A, Kinoshita Y, et al. An anti–glypican 3/CD3 bispecific T cell–redirecting antibody for treatment of solid tumors. Sci Transl Med. 2017;9:eaal4291.
-
Moore PA, Shah K, Yang Y, Alderson R, Roberts P, Long V, et al. Development of MGD007, a gpA33 x CD3-bispecific DART protein for T-cell immunotherapy of metastatic colorectal cancer. Mol Cancer Ther. 2018;17:1761–72.
-
Hernandez-Hoyos G, Sewell T, Bader R, Bannink J, Chenault RA, Daugherty M, et al. MOR209/ES414, a novel bispecific antibody targeting PSMA for the treatment of metastatic castration-resistant prostate cancer. Mol Cancer Ther. 2016;15:2155–65.
-
Xu H, Cheng M, Guo H, Chen Y, Huse M, Cheung NV. Retargeting T cells to GD2 pentasaccharide on human tumors using bispecific humanized antibody. Cancer Immunol Res. 2015;3:266–77.
-
Gan HK, Cvrljevic AN, Johns TG. The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS J. 2013;280:5350–70.
-
Aldoss I, Song J, Stiller T, Nguyen T, Palmer J, O'Donnell M, et al. Correlates of resistance and relapse during blinatumomab therapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol. 2017;92:858–65.
-
Zhao Y, Aldoss I, Qu C, Crawford JC, Gu Z, Allen EK, et al. Tumor-intrinsic and -extrinsic determinants of response to blinatumomab in adults with B-ALL. Blood. 2021;137:471–84.
-
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. New Engl J Med. 2018;378:449–59.
-
King AC, Bolanos R, Velasco K, Tu H, Zaman F, Geyer MB, et al. Real world chart review of blinatumomab to treat patients with high disease burden of relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Blood. 2019;134:5079.
-
Brown P, Zugmaier G, Gore L, Tuglus CA, Stackelberg A. Day 15 bone marrow minimal residual disease predicts response to blinatumomab in relapsed/refractory paediatric B-ALL. Brit J Haematol. 2019;188:e36–9.
-
Walker AJ, Majzner RG, Zhang L, Wanhainen K, Long AH, Nguyen SM, et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol Ther. 2017;25:2189–201.
-
Watanabe K, Terakura S, Martens AC, van Meerten T, Uchiyama S, Imai M, et al. Target antigen density governs the efficacy of anti–CD20-CD28-CD3 ζ chimeric antigen receptor–modified effector CD8+ T cells. J Immunol. 2015;194:911–20.
-
Krupka C, Kufer P, Kischel R, Zugmaier G, Lichtenegger FS, Köhnke T, et al. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: reversing a T-cell-induced immune escape mechanism. Leukemia. 2016;30:484–91.
-
Yuraszeck T, Bartlett D, Singh I, Reed M, Pagano S, Zhu M. A quantitative systems pharmacology (QSP) model to assess the action of blinatumomab in NHL patients (pts). J Clin Oncol. 2016;34:e14511.
-
Duell J, Dittrich M, Bedke T, Mueller T, Eisele F, Rosenwald A, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia. 2017;31:2181–90.
-
Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016:567–72.
-
Stein AS, Schiller G, Benjamin R, Jia C, Zhang A, Zhu M, et al. Neurologic adverse events in patients with relapsed/refractory acute lymphoblastic leukemia treated with blinatumomab: management and mitigating factors. Ann Hematol. 2019;98:159–67.
-
Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6:56.
-
Zheng P, Kros JM, Wang G. Elusive neurotoxicity in T cell-boosting anticancer therapies. Trends Immunol. 2019;40:274–8.
-
Klinger M, Zugmaier G, Nägele V, Goebeler M, Brandl C, Stelljes M, et al. Adhesion of T cells to endothelial cells facilitates blinatumomab-associated neurologic adverse events. Cancer Res. 2020;80:91–101.
-
Matasar MJ, Cheah CY, Yoon DH, Assouline SE, Bartlett NL, Ku M, et al. Subcutaneous mosunetuzumab in relapsed or refractory B-cell lymphoma: promising safety and encouraging efficacy in dose escalation cohorts. Blood. 2020;136:45–6.
-
Lesokhin AM, Levy MY, Dalovisio AP, Bahlis NJ, Solh M, Sebag M, et al. Preliminary safety, efficacy, pharmacokinetics, and pharmacodynamics of subcutaneously (SC) administered PF-06863135, a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma (RRMM). Blood. 2020;136:8–9.
-
Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–5.
-
Köhnke T, Krupka C, Tischer J, Knösel T, Subklewe M. Increase of PD-L1 expressing B-precursor ALL cells in a patient resistant to the CD19/CD3-bispecific T cell engager antibody blinatumomab. J Hematol Oncol. 2015;8:111.
-
Feucht J, Kayser S, Gorodezki D, Hamieh M, Döring M, Blaeschke F, et al. T-cell responses against CD19+ pediatric acute lymphoblastic leukemia mediated by bispecific T-cell engager (BiTE) are regulated contrarily by PD-L1 and CD80/CD86 on leukemic blasts. Oncotarget. 2016;7:76902–19.
-
Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–44.
-
Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019;20:1100–9.
-
Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–44.
-
Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5:3–8.
-
Perez C, Botta C, Zabaleta A, Puig N, Cedena M, Goicoechea I, et al. Immunogenomic identification and characterization of granulocytic myeloid-derived suppressor cells in multiple myeloma. Blood. 2020;136:199–209.
-
Jabbour E, Düll J, Yilmaz M, Khoury JD, Ravandi F, Jain N, et al. Outcome of patients with relapsed/refractory acute lymphoblastic leukemia after blinatumomab failure: no change in the level of CD19 expression. Am J Hematol. 2018;93:371–4.
-
Yu H, Sotillo E, Harrington C, Wertheim G, Paessler M, Maude SL, et al. Repeated loss of target surface antigen after immunotherapy in primary mediastinal large B cell lymphoma. Am J Hematol. 2017;92:E11–3.
-
Bukhari A, El Chaer F, Koka R, Singh Z, Hutnick E, Ruehle K, et al. Rapid relapse of large B-cell lymphoma after CD19 directed CAR-T-cell therapy due to CD-19 antigen loss. Am J Hematol. 2019;94:E273–5.
-
Braig F, Brandt A, Goebeler M, Tony H, Kurze A, Nollau P, et al. Resistance to anti-CD19/CD3 BiTE in acute lymphoblastic leukemia may be mediated by disrupted CD19 membrane trafficking. Blood. 2017;129:100–4.
-
Zhang Z, Chen X, Tian Y, Li F, Zhao X, Liu J, et al. Point mutation in CD19 facilitates immune escape of B cell lymphoma from CAR-T cell therapy. J Immunother Cancer. 2020;8:e001150.
-
Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5:1282–95.
-
Gardner R, Wu D, Cherian S, Fang M, Hanafi L, Finney O, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127:2406–10.
-
Rayes A, McMasters RL, O'Brien MM. Lineage switch in MLL-rearranged infant leukemia following CD19-directed therapy. Pediatr Blood Cancer. 2016;63:1113–5.
-
Zoghbi A, Winkler B, Zur Stadt U, Müller I, Escherich G. Lineage switch under blinatumomab of a relapsed common ALL co-expressing myeloid markers without MLL rearrangement. Blood. 2016;128:5196.
-
Oberley MJ, Gaynon PS, Bhojwani D, Pulsipher MA, Gardner RA, Hiemenz MC, et al. Myeloid lineage switch following chimeric antigen receptor T-cell therapy in a patient with TCF3-ZNF384 fusion-positive B-lymphoblastic leukemia. Pediatr Blood Cancer. 2018;65:e27265.
-
He RR, Nayer Z, Hogan M, Cuevo RS, Woodward K, Heyer D, et al. Immunotherapy- (blinatumomab-) related lineage switch of KMT2A/AFF1 rearranged B-lymphoblastic leukemia into acute myeloid leukemia/myeloid sarcoma and subsequently into B/myeloid mixed phenotype acute leukemia. Case Rep Hematol. 2019;2019:7394619.
-
Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126:3814–26.
-
Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13:30.
-
Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69:4941–4.
-
Lorenczewski G, Friedrich M, Kischel R, Dahlhoff C, Anlahr J, Balazs M, et al. Generation of a half-life extended anti-CD19 BiTE® antibody construct compatible with once-weekly dosing for treatment of CD19-positive malignancies. Blood. 2017;130:2815.
-
Marion S, Anthony S, Roland BW, Ravi B, Andrew HW, David R, et al. Updated results from a phase 1 first-in-human dose-escalation study of AMG 673, a novel anti-CD33/CD3 BiTE® (bispecific T-cell engager) molecule in patients with relapsed/refractory (R/R) acute myeloid leukemia (AML). EHA Library. 2020;294466:EP548.
-
Herrmann M, Krupka C, Deiser K, Lindl B, Mocikat R, Metzeler KH, et al. Development of a bifunctional checkpoint inhibitory T cell engager (CiTE) to reverse adaptive immune escape in AML. Blood. 2018;132:4069.
-
Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–56.
-
Liu R, Jiang W, Yang M, Guo H, Zhang Y, Wang J, et al. Efficient inhibition of human B-cell lymphoma in SCID mice by synergistic antitumor effect of human 4-1BB ligand/anti-CD20 fusion proteins and anti-CD3/anti-CD20 diabodies. J Immunother. 2010;33:500–9.
-
Arndt C, Feldmann A, von Bonin M, Cartellieri M, Ewen E, Koristka S, et al. Costimulation improves the killing capability of T cells redirected to tumor cells expressing low levels of CD33: description of a novel modular targeting system. Leukemia. 2014;28:59–69.
-
Laszlo GS, Gudgeon CJ, Harrington KH, Walter RB. T-cell ligands modulate the cytolytic activity of the CD33/CD3 BiTE antibody construct, AMG 330. Blood Cancer J. 2015;5:e340.
-
Correnti CE, Laszlo GS, de van der Schueren WJ, Godwin CD, Bandaranayake A, Busch MA, et al. Simultaneous multiple interaction T-cell engaging (SMITE) bispecific antibodies overcome bispecific T-cell engager (BiTE) resistance via CD28 co-stimulation. Leukemia. 2018;32:1239–43.
-
Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37:1049–58.
-
Guo ZS, Lotze MT, Zhu Z, Storkus WJ, Song X. Bi- and tri-specific T cell engager-armed oncolytic viruses: next-generation cancer immunotherapy. Biomedicines. 2020;8:204.
-
Scott EM, Jacobus EJ, Lyons B, Frost S, Freedman JD, Dyer A, et al. Bi- and tri-valent T cell engagers deplete tumour-associated macrophages in cancer patient samples. J Immunother Cancer. 2019;7:320.
-
Freedman JD, Duffy MR, Lei-Rossmann J, Muntzer A, Scott EM, Hagel J, et al. An oncolytic virus expressing a T-cell engager simultaneously targets cancer and immunosuppressive stromal cells. Cancer Res. 2018;78:6852–65.
-
Porter CE, Rosewell Shaw A, Jung Y, Yip T, Castro PD, Sandulache VC, et al. Oncolytic adenovirus armed with BiTE, cytokine, and checkpoint inhibitor enables CAR T cells to control the growth of heterogeneous tumors. Mol Ther. 2020;28:1251–62.
-
Klupsch K, Baeriswyl V, Scholz R, Dannenberg J, Santimaria R, Senn D, et al. Abstract 1787: COVA4231, a potent CD3/CD33 bispecific FynomAb with IgG-like pharmacokinetics for the treatment of acute myeloid leukemia. Cancer Res. 2018;78:1787.
-
Liu L, Lam CK, Long V, Widjaja L, Yang Y, Li H, et al. MGD011, A CD19 x CD3 dual-affinity retargeting bi-specific molecule incorporating extended circulating half-life for the treatment of B-cell malignancies. Clin Cancer Res. 2017;23:1506–18.
-
Mayes P, Tacken P, Wang S, Loo PV, Condamine T, Maaden HVD, et al. Abstract 539: a bispecific Fc-silenced IgG1 antibody (MCLA-145) requires PD-L1 binding to activate CD137. Cancer Res. 2019;79:539.
-
Seckinger A, Delgado JA, Moser S, Moreno L, Neuber B, Grab A, et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. 2017;31:396–410.
-
Schlothauer T, Herter S, Koller CF, Grau-Richards S, Steinhart V, Spick C, et al. Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Eng Des Sel. 2016;29:457–66.
-
Chornoguz O, Leettola CN, Leander K, Brosnan K, Emmell E, Chiu ML, et al. Characterization of a novel bispecific antibody that activates T cells in vitro and slows tumor growth in vivo. Monoclon Antib Immunodiagn Immunother. 2019;38:242–54.
-
Engelberts PJ, Hiemstra IH, de Jong B, Schuurhuis DH, Meesters J, Beltran Hernandez I, et al. DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. Ebiomedicine. 2020;52:102625.
-
Wang L, Hoseini SS, Xu H, Ponomarev V, Cheung N. Silencing fc domains in T cell–engaging bispecific antibodies improves T-cell trafficking and antitumor potency. Cancer Immunol Res. 2019;7:2013–24.
-
Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–19.
-
Wu L, Seung E, Xu L, Rao E, Lord DM, Wei RR, et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat Can. 2020;1:86–98.
-
Seftel MD. Hyper-CVAD: a regimen for all seasons. Lancet Haematol. 2020;7:e501–2.
-
Richard-Carpentier G, Kantarjian HM, Short NJ, Ravandi F, Ferrajoli A, Schroeder HM, et al. Updated results from the phase II study of hyper-CVAD in sequential combination with blinatumomab in newly diagnosed adults with B-cell acute lymphoblastic leukemia (B-ALL). Blood. 2019;134:3807.
-
Fleming S, Venn N, Reynolds J, Nguyen U, Kwan J, Moore J, et al. Preliminary minimal residual disease analysis of the Australasian Leukaemia & Lymphoma Group (ALLG) ALL8 study of front-line blinatumomab with chemotherapy in adults with Ph negative B-cell acute lymphoblastic leukaemia. Blood. 2019;134:1300.
-
Short NJ, Kantarjian HM, Ravandi F, Huang X, Ferrajoli A, Kadia TM, et al. Hyper-CVAD and sequential blinatumomab in adults with newly diagnosed Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia: results from a phase II study. Blood. 2020;136:9–11.
-
Katz DA, Chu MP, David KA, Thieblemont C, Morley NJ, Khan SS, et al. Open-label, phase 2 study of blinatumomab after first-line rituximab-chemotherapy in adults with newly diagnosed, high-risk diffuse large B-cell lymphoma. Blood. 2019;134:4077.
-
Sasaki K, Kantarjian HM, Ravandi F, Short NJ, Kebriaei P, Huang X, et al. Sequential combination of inotuzumab ozogamicin (InO) with low-intensity chemotherapy (mini-hyper-CVD) with or without blinatumomab is highly effective in patients (pts) with Philadelphia chromosome-negative acute lymphoblastic leukemia (ALL) in first relapse. Blood. 2019;134:3806.
-
Sasaki K, Kantarjian HM, Ravandi F, Short NJ, Kebriaei P, Huang X, et al. Long-term follow-up of the combination of low-intensity chemotherapy plus inotuzumab ozogamicin with or without blinatumomab in patients with relapsed-refractory Philadelphia chromosome-negative acute lymphoblastic leukemia: a phase 2 trial. Blood. 2020;136:40–2.
-
McCloskey JK, Gagnon J, McCabe T, Charlon J, Wang S, Fan R, et al. Blinatumomab in combination with tyrosine kinase inhibitors safely and effectively induces rapid, deep, and durable molecular responses in relapsed and refractory Philadelphia positive acute leukemias. Blood. 2019;134:3812.
-
Assi R, Kantarjian H, Short NJ, Daver N, Takahashi K, Garcia-Manero G, et al. Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed Philadelphia chromosome-positive leukemia. Clin Lymphoma Myeloma Leuk. 2017;17:897–901.
-
Couturier M, Thomas X, Huguet F, Berthon C, Simand C, Hicheri Y, et al. Blinatumomab + ponatinib for relapsed Ph1-positive acute lymphoblastic leukemia: the French experience. Blood. 2018;132:4014.
-
Chiaretti S, Bassan R, Vitale A, Elia L, Piciocchi A, Puzzolo C, et al. Dasatinib-blinatumomab combination for the front-line treatment of adult Ph+ ALL patients. Updated results of the gimema LAL2116 D-Alba trial. Blood. 2019;134:740.
-
Campos-Cabrera G, Vega-Tapia N, Campos-Cabrera V, Campos-Cabrera S, Campos-Villagomez J, Mendez-Garcia E. Blinatumomab and venetoclax for minimal residual disease relapse in acute lymphoblastic leukemia. Blood. 2019;134:5128.
-
Webster J, Luskin MR, Prince GT, DeZern AE, DeAngelo DJ, Levis MJ, et al. Blinatumomab in combination with immune checkpoint inhibitors of PD-1 and CTLA-4 in adult patients with relapsed/refractory (R/R) CD19 positive B-cell acute lymphoblastic leukemia (ALL): preliminary results of a phase I study. Blood. 2018;132:557.
-
Schwartz M, Damon LE, Jeyakumar D, Costello CL, Tzachanis D, Schiller GJ, et al. Blinatumomab in combination with pembrolizumab is safe for adults with relapsed or refractory B-lineage acute lymphoblastic leukemia: University of California Hematologic Malignancies Consortium Study 1504. Blood. 2019;134:3880.
-
Buccheri S, Guggino G, Caccamo N, Li DP, Dieli F. Efficacy and safety of gammadeltaT cell-based tumor immunotherapy: a meta-analysis. J Biol Regul Homeost Agents. 2014;28:81–90.
-
Sebestyen Z, Prinz I, Dechanet-Merville J, Silva-Santos B, Kuball J. Translating gammadelta (gammadelta) T cells and their receptors into cancer cell therapies. Nat Rev Drug Discov. 2020;19:169–84.
-
de Bruin RCG, Veluchamy JP, Lougheed SM, Schneiders FL, Lopez-Lastra S, Lameris R, et al. A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vγ9Vδ2-T cells. Oncoimmunology. 2017;7:e1375641.
-
Kuhns MS, Badgandi HB. Piecing together the family portrait of TCR-CD3 complexes. Immunol Rev. 2012;250:120–43.
-
Bachiller M, Battram AM, Perez-Amill L, Martín-Antonio B. Natural killer cells in immunotherapy: are we nearly there? Cancers. 2020;12:3139.
-
Schmohl JU, Felices M, Taras E, Miller JS, Vallera DA. Enhanced ADCC and NK cell activation of an anticarcinoma bispecific antibody by genetic insertion of a modified IL-15 cross-linker. Mol Ther. 2016;24:1312–22.
-
Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU, et al. IL15 trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin Cancer Res. 2016;22:3440–50.
-
Gauthier L, Morel A, Anceriz N, Rossi B, Blanchard-Alvarez A, Grondin G, et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell. 2019;177:1701–1713.e16.
-
Heczey A, Courtney AN, Montalbano A, Robinson S, Liu K, Li M, et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med. 2020;26:1686–90.
-
Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38:947–53.
Acknowledgements
I would like to thank my supervisor, Dr. Meng, for her guidance through each stage of the process.
Funding
The study was funded by the National Natural Science Foundation of China (No.81972796 and 81972863), the National Key Research and Development Project (2018YFC1313200), the Innovation Project of Shandong Academy of Medical Sciences (2019–04), the Natural Science Foundation of Shandong (No.ZR2019MH010), and the Academic Promotion Program of Shandong First Medical University (2019ZL002).
Author information
Authors and Affiliations
Contributions
Shujie Zhou: Contributed to Writing - Original Draft, Investigation, and Resources. Mingguo Liu: Contributed to Resources. Fei Ren: Contributed to Resources. Jinming Yu: Contributed to the Writing - Review & Editing and Funding acquisition. Xiangjiao Meng: Contributed to Study design, Supervision, and Writing - Review & Editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Gerelateerde artikelen
- Coronavaccin stimuleert effectiviteit van immuuntherapie en verlengt overleving bij kankerpatienten indien gegeven gelijktijdig of vlak erna naast immuuntherapie
- Oorzaak van spontane kankergenezing lijkt gevonden en het daarop algemene ontwikkelde mRNA vaccin werkte al effectief in dierstudies met melanomen, borstkanker en darmkanker door herprogrammering van afwijkende cellen
- Sucralose, een kunstmatige zoetstof, vermindert effectiviteit van immuuntherapie en geeft slechtere overall overleving bij patienten met verschillende vormen van kanker, waaronder longkanker en melanomen
- Akkermansia muciniphila, een probiotica bacterie, blijkt de effectiviteit van immuuntherapie bij kankerpatienten sterk te verbeteren, blijkt uit meerdere studies
- Oncolytische virussen geven uitstekende resultaten in aanpak van kanker. Clemens Dirven en Casper van Eyck vertellen in Op1 over hun nieuwste vinding waarmee patient met hersentumor is genezen
- Immuuntherapie met gemoduleerde navelstreng natural killer cellen - UBC-nk celtherapie. Wat is dat voor vorm van immuuntherapie?
- Immuuntherapie met anti-PD medicijnen met lagere doses bij kankerpatienten geeft therapeutisch gezien zelfde goede effect als standaard dosering maar veel minder bijwerkingen en kosten zijn veel minder.
- BiTE = Bispecifieke T-cel-engager blijkt hoopvolle nieuwe variant van immuuntherapie met gemoduleerde CAR-T cellen bij de behandeling van kanker. Hier een reviewstudie
- Onderzoekers ontdekken ‘schakelaar’ om apoptose = geprogrammeerde celdood van kankercellen te activeren en lijkt heel belangrijk voor CAR-T celtherapie bij solide tumoren
- Photo Immuno Therapy (PIT) = PDT met infrarood licht blijkt veelbelovende vorm van immuuntherapie voor solide tumoren al of niet in combinatie met andere behandelingen
- Coley vaccin: Immuuntherapie met het Coley vaccin (koorts therapie) stond aan de basis van de huidige immuuntherapie met gemoduleerde virussen en bacterien. Hier een reviewstudie van de Coley therapie
- Darmschimmels - darmbacterien beïnvloeden de immuunreactie na radiotherapie op positieve manier. Doden van darmbacterien door antibiotica heeft negatief effect op immuunreacties na bestraling copy 1
- Een petscan met Novel 18F-Labeled Adnectin kan direct laten zien of een kankerpatient voldoende PD-L1 Expressie heeft voor immuuntherapie met anti-PD medicijnen
- Het voordeel van immunotherapeutische combinatie behandelingen
- Het menselijk immuunsysteem bestaat uit aangeboren immuniteit en verworven immuniteit, maar hebben elkaar nodig voor een goed werkend immuunsysteem. Een uitleg
- Immuuntherapie bij kanker: Grootste doorbraak van 2013 op wetenschappelijk gebied, aldus vakblad Science
- Immuuntherapie met behulp van virussen en antigenen levert hele mooie resultaten op bij verschillende vormen van kanker.
- NLR meting - veranderende verhouding van neutrofielen tot lymfocyten - blijkt een uitstekende en eenvoudige manier om de werkzaamheid van immuuntherapie met anti-PD medicijnen tijdens behandelingsfase te controleren.
- Overzicht van alle wereldwijd geregistreerde medicijnen binnen immuuntherapie en lopende studies met immuuntherapie
- Radiotherapy and MVA-MUC1-IL-2 vaccine act synergistically for inducing specific immunity to MUC-1 tumor antigen
- Rigvir:Immuuntherapie met het gemoduleerde Rigvir virus blijkt bij verschillende vormen van kanker beduidend betere resultaten te geven op overall overleving en complete remissies - genezingen. Een overzichtsstudie van de ontwikkeling van het RIGVIR virus
- Slapende stamcellen: immuuntherapie met oncolytische virussen om recidief te voorkomen via de slapende kankerstamcellen blijkt veelbelovende aanpak. Hier een overzichtsstudie van stand van zaken met deze vorm van immuuntherapie
- Vaccins tegen kanker: Molecuul PRIMA-1 lijkt beschadigde gen P53 tot op DNA niveau te herstellen.
- Virussen: Bewerkt adenovirus - E1B 19kD - rechtstreeks ingebracht in tumorweefsel zorgt voor opmerkelijke resultaten.
- Wat is de juiste patient voor immuuntherapie? Nederlands onderzoek komt tot opmerkelijke conclusies. Internist-oncoloog Hans Westgeest geeft uitleg
- Algemene artikelen over immuuntherapie



Plaats een reactie ...
Reageer op "BiTE = Bispecifieke T-cel-engager blijkt hoopvolle nieuwe variant van immuuntherapie met gemoduleerde CAR-T cellen bij de behandeling van kanker. Hier een reviewstudie"