Helpt u ons aan 500 donateurs?
Vooraf nog een keer gezegd: het mechanisme van het Novocure - NovoTTF™-100A System is vergelijkbaar met de werking van electro hyperthermie. Of patiënten via hun behandelend arts een TTF apparaatje kunnen gebruiken / verkrijgen durf ik niet te zeggen maar lijkt de moeite waard het te proberen. Of via uw ziektekostenverzekeraar wellicht? De onderzoeksleider is notabene een Nederlandse oncoloog:
Corresponding Author: Martin J. B. Taphoorn, MD, PhD, Department of Neurology, Haaglanden Medical Center, PO BOX 2191, 2501 VC, The Hague, The Netherlands (m.taphoorn@haaglandenmc.nl).
24 februari 2018: Bron: JAMA Oncology
Uit een aanvullende analyse van de fase III studie, zie ook hieronder, blijkt dat met het Novocure - NovoTTF™-100A System het algemeen bijwerkingenprofiel niet verergert maar op sommige punten juist verbetert.
-
In deze secundaire analyse van de EF-14 gerandomiseerde klinische studie had de toevoeging van tumorbehandelingsvelden geen negatieve invloed op de gezondheidsgerelateerde kwaliteit van leven, behalve een jeukende huid, een te verwachten gevolg van de apparaatjes op het hoofd. Van tumorbehandelingstherapie is eerder aangetoond dat het zowel de progressievrije als de totale overleving verlengt. (zie hieronder resultaten uit fase III studie)
-
Naast een klinisch voordeel van verbeterde mediane overleving met ca. 5 maanden wordt deze verbeterde overleving ook ondersteunt zonder dat het een negatieve invloed heeft op de gezondheidsgerelateerde kwaliteit van leven door de toevoeging van tumorbehandelingsvelden (TTR fields) aan een standaardbehandeling bij patiënten met hersentumoren van het type glioblastoma.
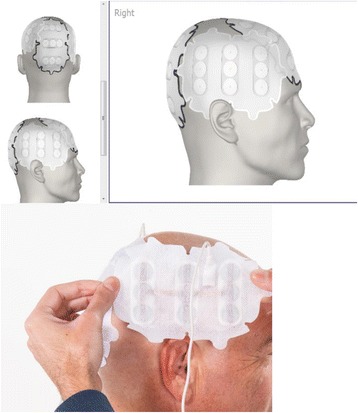 NOVO TTF-100A
NOVO TTF-100A
Het originele studierapport van deze tweede analyse: Influence of Treatment With Tumor-Treating Fields on Health-Related Quality of Life of Patients With Newly Diagnosed GlioblastomaA Secondary Analysis of a Randomized Clinical Trial is gratis in te zien met een gedetailleerde beschrijving van manier van analyseren en de resultaten op verschillende bijwerkingen. (Abstract staat onderaan dit artikel)
Nog een keer gezegd: het mechanisme van het Novocure - NovoTTF™-100A System is vergelijkbaar met de werking van electro hyperthermie. Of patiënten via hun behandelend arts een TTF apparaatje kunnen gebruiken / verkrijgen durf ik niet te zeggen maar lijkt de moeite waard het te proberen. Of via uw ziektekostenverzekeraar wellicht? De onderzoeksleider is notabene een Nederlandse oncoloog:
Corresponding Author: Martin J. B. Taphoorn, MD, PhD, Department of Neurology, Haaglanden Medical Center, PO BOX 2191, 2501 VC, The Hague, The Netherlands (m.taphoorn@haaglandenmc.nl).
30 december 2017: Het uiteindelijke studierapport: Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. met de definitieve resultaten is afgelopen week online gepubliceerd.
Resutlaten laten een statistische mediane progressievrije overleving zien van 2,7 maanden met temodal plus de NOVO TTF behandeling in vergelijking met een behandeling met alleen temodal bij hersentumoren van het type glioblastoma multiuforme nadat deze allemaal een operatie plus bestraling hadden ondergaan. Mediane overall overleving was 4,9 maanden beter (20,9 maanden versus 16 maanden). Ca. 25 procent levensverlenging is toch wel veel voor deze vorm van bijna altijd dodelijke vorm van kanker.
Resultaten:
Van de 695 gerandomiseerd ingedeelde patiënten (mediane leeftijd, 56 jaar, IQR, 48-63; 473 mannen [68%]), voltooiden 637 patiënten (92%) het onderzoek. De mediane progressievrije overleving na randomisatie was 6,7 maanden in de TTFields-temozolomide-groep en 4,0 maanden in de temozolomide-alleen-groep (HR, 0,63; 95% CI, 0,52-0,76; P <0,001). De mediane totale overleving was 20,9 maanden in de TTFields-temozolomide-groep versus 16,0 maanden in de temozolomide-alleen-groep (HR, 0,63; 95% CI, 0,53-0,76; P <0,001). Bijwerkingenprofiel was 48% in de TTFields-temozolomide-groep en 44% in de temozolomide-alleen-groep. Milde tot matige huidtoxiciteit onder de transducer-arrays trad op bij 52% van de patiënten die TTFields-temozolomide kregen versus geen patiënten die temozolomide alleen kregen.
Conclusies en relevantie:
In de uiteindelijke analyse van deze gerandomiseerde klinische studie van patiënten met glioblastoma die standaard radiochemotherapie hadden ontvangen, resulteerde de toevoeging van TTFields aan onderhoudsbehandeling van temozolomide-chemotherapie versus onderhoudsbehandeling van temozolomide alleen in statistisch significante verbetering van progressievrije overleving en totale overleving. Deze resultaten komen overeen met de vorige tussentijdse analyse.
Zie verder hieronder de inforamtie die we afgelopen jaren hierover hebben gepubliceerd.
Results:
Of the 695 randomized patients (median age, 56 years; IQR, 48-63; 473 men [68%]), 637 (92%) completed the trial. Median progression-free survival from randomization was 6.7 months in the TTFields-temozolomide group and 4.0 months in the temozolomide-alone group (HR, 0.63; 95% CI, 0.52-0.76; P < .001). Median overall survival was 20.9 months in the TTFields-temozolomide group vs 16.0 months in the temozolomide-alone group (HR, 0.63; 95% CI, 0.53-0.76; P < .001). Systemic adverse event frequency was 48% in the TTFields-temozolomide group and 44% in the temozolomide-alone group. Mild to moderate skin toxicity underneath the transducer arrays occurred in 52% of patients who received TTFields-temozolomide vs no patients who received temozolomide alone.
Conclusions and Relevance:
In the final analysis of this randomized clinical trial of patients with glioblastoma who had received standard radiochemotherapy, the addition of TTFields to maintenance temozolomide chemotherapy vs maintenance temozolomide alone, resulted in statistically significant improvement in progression-free survival and overall survival. These results are consistent with the previous interim analysis.
3 mei 2017: Bron: ASCO 2017 en World J Surg Oncol. 2015; 13: 316
De Novocure - NovoTTF™-100A System, een door een draagbare batterij aangedreven apparaatje (een soort van koptelefoon) dat zogeheten TTF - Tumor Treating Fields - alternerende elektrische velden (electro hyperthermie in feite) levert via elektroden die aan de hoofdhuid zijn bevestigd, verlengt de overall overleving van patiënten met een nieuwe diagnose van een hersentumor van het type glioblastoom met 5 maanden: 20,9 vs 16 maanden. Dit blijkt uit de eindresultaten van een fase 3 studie met totaal 695 patiënten met een eerste diagnose van een hersentumor: voor de Novocure (n = 466) plus TMZ - temodal en controlegroep van (N = 229) met alleen temozolomide - TMZ als behandeling na operatie en/of bestraling. De Novocure wordt dus door patienten gewoon thuis gebruikt. (zie ook dit artikel dat we eerder schreven over de Novocure waarin ervaringsverhaal van man met de Novocure)
Of lees dit review artikel: Tumor treating fields: a novel treatment modality and its use in brain tumors.
Conclusions & Future Perspectives
The results of the randomized phase III EF-14 trial provide level 1 evidence that alternating electric fields are able to positively impact tumor growth and significantly extend survival in GBM. As a logical consequence, TTFields were approved by the FDA for newly diagnosed GBM in October 2015. Nevertheless, numerous questions remain and need to be addressed, both within the EF-14 trial and in future studies; Notably, it will be essential to be able to (1) identify the patients most likely to respond to TTFields therapy, (2) further elucidate the mechanism of action of TTFields, (3) elucidate the mechanisms of resistance to and failure of TTFields therapy, and (4) elucidate the pattern and predictors of response.
Fig. 1

Electric field magnitude and distribution (in V/cm) shown in coronal view from a finite element method simulation model. This simulation employs a left-right paired transducer array configuration. Reprinted with permission from Miranda et al. [5]
Deze resultaten bevestigen de eerder vrijgegeven tussenresultaten die de basis vormden voor de FDA goedkeuring van het Novocure apparaat voor de eerstelijns behandeling van een glioblastoom in 2015. En deze nieuwe publicatie bevestigt ook hiermee de resultaten uit een studie uitgevoerd bij patienten met een recidief van een glioblastoma na standaard eerstelijns behandeling.
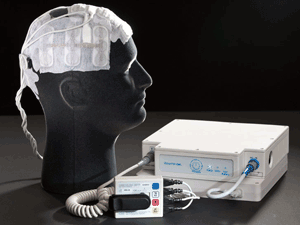 |
| The NovoTTF-100A system |
In March 2010, the Neurological Devices Panel of the Medical Devices Advisory Committee voted 7 to 3 (with 2 abstentions) that, overall, the benefits of NovoTTF outweigh the risks in recurrent GBM.
De onderzoekers hopen dat de nieuwe, definitieve gegevens die hier op AACR 2017 werden gepresenteerd, zullen bijdragen aan de verbetering van het imago van de Novocure want in de ogen van oncologen en neuro chirurgen is de Novocure een vreemde kwakzalverachtig aandoende behandeling en is nog steeds niet volledig geaccepteerd ondanks dat de FDA al in 2010 de novocure goedkeurde. Laat staan dat behandelend artsen hun patienten dit aan zouden raden. Waar hebben we dit eerder gehoord? Tekenend is dat Medscape het grootste medische platform in Amerika deze studieresultaten beschrijft onder de titel: It's 'Wacky' but Improves Survival in Glioblastoma
Hier een tekening van de Novocure: (tekst gaat verder onder tekening)
Fig. 2

Sample transducer array layout map guiding placement of transducer arrays on the scalp
Volgens hoofdonderzoeker Roger Stupp, MD, een neuro-oncoloog aan de Northwestern University in Chicago, Illinois, legde uit dat het overlevingsvoordeel dat door de Novocure wordt gerealiseerd de eerste significante verbetering is nadat temodal - temozolomide in (TMZ) (Temodar, Merck) goedgekeurd is in 2005.
De mediane overall overleving is vergelijkbaar met die van temodal - temozolomide. Samen verlengt het de overall overleving statistisch significant.
De mediane algehele overleving voor de Novocure - TTF / temodal - TMZ groep was 20,9 maanden, versus 16,0 maanden voor temodal - TMZ alleen (HR, 0,63; P = .00006).
Op 2 jaars meting leefde 43,1% van de TTF / TMZ-groep nog steeds, tegenover 37,0% van de TMZ-alleengroep.
Interessant is ook deze studie: NovoTTF™-100A System (Tumor Treating Fields) transducer array layout planning for glioblastoma: a NovoTAL™ system user study waarin artsen / oncologen van buiten Novocure werd gevraagd om scans te beoordelen enz. En vergeleken met de beoordelingen van de artsen / oncol;goen die bij de producent van Novocure werkten.
Ook deze studie: Planning TTFields treatment using the NovoTAL system-clinical case series beyond the use of MRI contrast enhancement. beschrijft dit proces.
Hier de studies die lopen met het NovoTTF™-100A System d.d. februari 2017 en dus niet alleen bij hersentumoren maar ook bij andere vormen van kanker:
Table 2.
Ongoing clinical trials in solid tumors
| Disease, indication | Name of trial | Protocol description | # Pts | Phase | Endpoints | Sponsor | NCT# |
|---|---|---|---|---|---|---|---|
| Recurrent GBM | |||||||
| Recurrent GBM | EF-26 (Japan) | Prospective, single arm, multicenter, postapproval study of TTFields in recurrent GBM patients | 30 | IV | Incidence & severity of treatment- related skin & CNS disorders: secondary: 1-y survival; PFS6mo | Novocure (EF-26) (Japan) | NA (Japan) |
| Recurrent GBM, bev-naive | Optune™+ bev & hypofractionated stereotactic RT in bev- naive GBM | TTFields + bev + SRT in recurrent GBM | 27 | I | Safety | U Maryland | NCT01925573 |
| Recurrent GBM, at first relapse | Optune™ with bev & carmustine in treating patients with GBM in first relapse | TTFields+ bev + BCNU | 20 | II, single arm | Safety, PFS, PFS6, OS | UC Davis | NCT02348255 |
| Recurrent bev- refractory GBM | Multicenter study of TTFields & pulsed bev | TTFields + pulsed Bev | 25 | II, single arm | PFS, QoL questionnaires | Univ of Florida | NCT02663271 |
| Recurrent GBM | Phase 2 study of TTFields, enhanced by genomic analysis to identify a genetic signature for response | TTFields | 30 | II, pilot | Efficacy; QoL | Washington Univ School of Medicine | NCT01954576 |
| Recurrent GBM | Optune™ with bev in GBM | TTFields + bev | 40 | II, open label | Safety & Efficacy | Case Comprehensive Cancer Ctr, Cleveland, OH | NCT01894061 Start- June 2013 End- April 2017 |
| Newly diagnosed & recurrent GBM | High Resolution MRI 7 MRS to Evaluate Therapeutic Response to Novo- TTF in Newly & Recurrent GBM | TTFields | 10 | II | RR, using SOC MRIs | Univ of Penn | NCT02441322 |
| Newly diagnosed GBM | |||||||
| Newly Diagnosed GBM, unresectable | A Phase II Study of Optune™ in combo with BEV &TMZ in pts with newly diagnosed unresectable GBM | RT + bev, followed by TTFields in combo with bev & TMZ | 46 | II, single arm | Safety & efficacy | Carolinas Healthcare System | NCT02343549 |
| Low grade gliomas | |||||||
| Newly diagnosed low grade gliomas | Phase II study of Optune™ ± TMZ in pts with Low- Grade Gliomas | TTFields ± TMZ | 42 | II, single arm | ORR, Secondary- PFS | UCSD | NCT02507232 |
| Meningioma | |||||||
| Recurrent Atypical & Anaplastic Meningioma | Pilot Study of Optune™ for Recurrent Atypical & Anaplastic Meningioma | TTFields | 21 | Pilot | Safety & Efficacy | MSKCC | NCT01892397 |
| Brain metastases | |||||||
| NSCLC with 1–5 brain metastases following optimal standard local treatment | COMET | Maintenance TTFields vs supportive care- best SOC alone (after completion of standard local therapy) | 60 | II, randomized | Time to local/ distant progression | Novocure (EF-21) EU | NCT01755624 |
| NSCLC with 1–10 brain metastases | METIS | Radiosurgery ± TTFields | 240 | III, randomized | Time to first cerebral progression | Novocure (EF-25) Global | Pending FDA IDE approval |
| Solid tumors | |||||||
| Advanced pancreatic adenocarcinoma | PANOVA | + gem or gem/nab- paclitaxel | 40 | I/II, 2-cohorts | Safety; feasibility; prelim efficacy | Novocure (EF-20) EU | NCT01971281 |
| Recurrent ovarian carcinoma | INNOVATE | Concomitant with weekly paclitaxel | 30 | I/II pilot study | Safety, toxicity, feasibility & prelim efficacy | Novocure (EF-22) EU | NCT02244502 |
| Pleural mesothelioma | STELLAR | Pemetrexed & cisplatin or carboplatin + TTFields | 80 | II, open label | Safety & efficacy (OS) | Novocure (EF-23) EU | NCT02397928 |
| Front-line treatment for advanced NSCLC with squamous histology | LUNAR | combination chemotherapy ± TTFields | 300 | pivotal open-label randomized | Protocol in preparation | Novocure (EF-24) Global | NA |
NCT#; ClinicalTrials.gov Identifier, Novocure; Novocure Ltd, manufacturer of TTFields (Optune™) device
Abbreviations: BCNU, carmustine;bev, bevacizumab (Avastin®); EU, European Union; GBM, glioblastoma; Gem, gemcitabine; MSKCC, Memorial Sloan-Kettering Cancer Center; nab-paclitaxel; albumin-bound paclitaxel (Abraxane®), NSCLC,non–small cell lung cancer; OS, overall survival; PFS, progression-free survival; EU
En hier de studiepublicatie uit 2015 in JAMA: Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. Met deze conclusie die nu dus in het eindrapprot wrodt bevestigd:
In this interim analysis of 315 patients with glioblastoma who had completed standard chemoradiation therapy, adding TTFields to maintenance temozolomide chemotherapy significantly prolonged progression-free and overall survival.
Hier het abstract van de studie met referentielijst
Conclusions In this interim analysis of 315 patients with glioblastoma who had completed standard chemoradiation therapy, adding TTFields to maintenance temozolomide chemotherapy significantly prolonged progression-free and overall survival.
Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial.
Abstract
IMPORTANCE:
Glioblastoma is the most devastating primary malignancy of the central nervous system in adults. Most patients die within 1 to 2 years of diagnosis. Tumor-treating fields (TTFields) are a locoregionally delivered antimitotic treatment that interferes with cell division and organelle assembly.
OBJECTIVE:
To evaluate the efficacy and safety of TTFields used in combination with temozolomide maintenance treatment after chemoradiation therapy for patients with glioblastoma.
DESIGN, SETTING, AND PARTICIPANTS:
After completion of chemoradiotherapy, patients with glioblastoma were randomized (2:1) to receive maintenance treatment with either TTFields plus temozolomide (n = 466) or temozolomide alone (n = 229) (median time from diagnosis to randomization, 3.8 months in both groups). The study enrolled 695 of the planned 700 patients between July 2009 and November 2014 at 83 centers in the United States, Canada, Europe, Israel, and South Korea. The trial was terminated based on the results of this planned interim analysis.
INTERVENTIONS:
Treatment with TTFields was delivered continuously (>18 hours/day) via 4 transducer arrays placed on the shaved scalp and connected to a portable medical device. Temozolomide (150-200 mg/m2/d) was given for 5 days of each 28-day cycle.
MAIN OUTCOMES AND MEASURES:
The primary end point was progression-free survival in the intent-to-treat population (significance threshold of .01) with overall survival in the per-protocol population (n = 280) as a powered secondary end point (significance threshold of .006). This prespecified interim analysis was to be conducted on the first 315 patients after at least 18 months of follow-up.
RESULTS:
The interim analysis included 210 patients randomized to TTFields plus temozolomide and 105 randomized to temozolomide alone, and was conducted at a median follow-up of 38 months (range, 18-60 months). Median progression-free survival in the intent-to-treat population was 7.1 months (95% CI, 5.9-8.2 months) in the TTFields plus temozolomide group and 4.0 months (95% CI, 3.3-5.2 months) in the temozolomide alone group (hazard ratio , 0.62 [98.7% CI, 0.43-0.89]; P = .001). Median overall survival in the per-protocol population was 20.5 months (95% CI, 16.7-25.0 months) in the TTFields plus temozolomide group (n = 196) and 15.6 months (95% CI, 13.3-19.1 months) in the temozolomide alone group (n = 84) (HR, 0.64 [99.4% CI, 0.42-0.98]; P = .004).
CONCLUSIONS AND RELEVANCE:
In this interim analysis of 315 patients with glioblastoma who had completed standard chemoradiation therapy, adding TTFields to maintenance temozolomide chemotherapy significantly prolonged progression-free and overall survival.
TRIAL REGISTRATION:
clinicaltrials.gov Identifier: NCT00916409.
Comment in
- PMID:
- 26670971
- DOI:
- 10.1001/jama.2015.16669
Use of TTFields prolongs progression-free and overall survival in patients with glioblastoma. The addition of this novel device-delivered treatment neither negatively affects nor improves functioning and well-being of the patient, including critical HRQOL issues, such as role, social, and physical functioning. Patients reported more itchy skin, which is a direct and expected consequence of the placement of transducer arrays on the patients’ scalp. Considering the net clinical benefit, our HRQoL data support the addition of TTFields to standard therapy in patients with glioblastoma.
Gerelateerde artikelen
- NovoTTF™-100A (TTF = vorm van electro hyperthermie) geeft veel langere mediane overleving, 5 maanden, bij nieuwe diagnose hersentumoren glioblastoma in vergelijking met temodal - temozolomide alleen
- NOVO TFF-100A: Fase III studie met electroden aanpak (NovoTTF-100A) van recidief van hersentumoren (Recidief van Glioblastoma Multiforme) geeft betere resultaten dan chemo.
- NovoTTF therapie - electrische pulsen - geeft vergelijkbare overlevingstijd dan met chemo bij patiënten met een recidief van een hersentumor glioblastoom multiforme. Kwaliteit van leven blijkt met de NOVO TTF veel beter.
- Behandeling met electrische pulsen geeft verdubbeling van overlevingstijd voor kankerpatienten met een inoperabel recidief van een hersentumor glioblastoma multiforma (GBM).
- FDA geeft officieel toestemming voor gebruik van het NovoTTF-100A systeem, electrische velden, bij hersentumoren glioblastomen multiforme.
- NOVO TFF-100A: een overzicht van artikelen



Plaats een reactie ...
Reageer op "NovoTTF™-100A (TTF = vorm van electro hyperthermie) geeft veel langere mediane overleving, 5 maanden, bij nieuwe diagnose hersentumoren glioblastoma in vergelijking met temodal - temozolomide alleen"