4 februari 2021: zie ook dit artikel: https://kanker-actueel.nl/zelflerende-algoritmen-leiden-tot-optimale-effectiviteit-van-door-hifu-ultra-sound-opgewekte-hyperthermie-bij-kwaadaardige-tumoren-aldus-daniel-deelen-in-zijn-proefschrift.html
In Nederland wordt in deze privekliniek HIFU toegepast bij prostaatkanker
Deze reviewstudie uit 2019 geeft overzicht van studies gedaan met HIFU wereldwijd.
20 november 2017: lees ook dit artikel:
6 november 2017: De verwijzingen naar de studies in het UMC van zowel Ultra Sound (MR-HIFU) bij borstkanker als bij botuitzaaiingen bij prostaatkanker (zie hieronder) geven geen website pagina meer. De studie bij borstkanker is afgesloten, die bij botmetastases waarschijnlijk ook want vind deze niet meer op de website van het UMC Utrecht en ook niet meer in clinical trials.
Maar voor de studie bij borstkanker zie deze publicatie:Nieuw onderzoek toont potentie MR-HIFU technologie voor nieuwe kankertherapie waarin de studie met borstkanker is verwerkt. Zie ook in dit verband deze studie: Nicole Hijnen et al., Thermal combination therapies for local drug delivery by magnetic resonance-guided high-intensity focused ultrasound, (PNAS, 31 mei 2017).
Een studie van Ultra Sound (MR-HIFU) bij botuitzaaiingen van de Nederlandse onderzoekers betrokken bij onderstaand omschreven onderzoek bij prostaatkankerpatiënten is deze: Quality of MR thermometry during palliative MR-guided high-intensity focused ultrasound (MR-HIFU) treatment of bone metastases
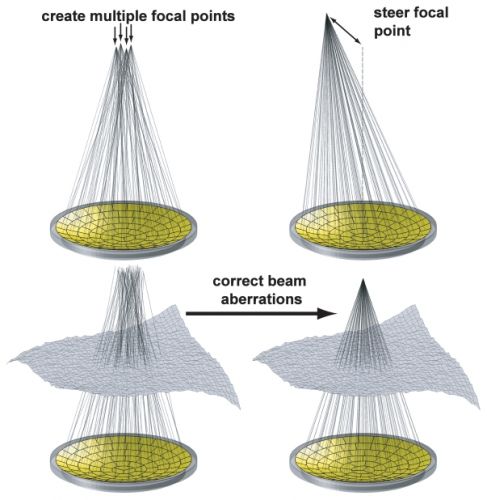
Het volledige studierapport is gratis in te zien met gedetailleerde omschrijvingen van hoe Ultra Sound werkt en ook grafieken van behandelde patienten. En uitstekende referentielijst.
Onderaan dit artikel staat abstract plus referentielijst
13 augustus 2013: Bron: UMC Utrecht
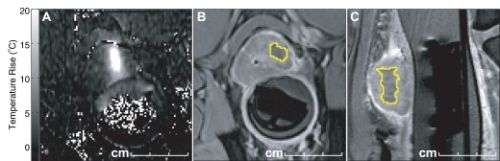
Foto: behandeling van botmetastase in bot via HIFU
Wat veel mensen niet weten en waar patiënten ook zelden op wordt gewezen is deze studie in het UMC Utrecht die al enkele jaren loopt en waarvoor nog steeds patiënten worden aangenomen, zie ook meer info over HIFU in gerelateerde artikelen:
MR-HIFU bij pijnlijke botuitzaaiingen
UMC Utrecht
Op deze website in dit PDF document staat in het Engels beschreven hoe de MRI-HIFU werkt: Klik hier voor dit document.
Quality of MR thermometry during palliative MR-guided high-intensity focused ultrasound (MR-HIFU) treatment of bone metastases
- Mie K LamEmail author,
- Merel Huisman,
- Robbert J Nijenhuis,
- Maurice AAJ van den Bosch,
- Max A Viergever,
- Chrit TW Moonen and
- Lambertus W Bartels
https://doi.org/10.1186/s40349-015-0026-7
© Lam et al.; licensee BioMed Central. 2015
Received: 21 November 2014
Accepted: 7 March 2015
Published: 24 March 2015
Abstract
Background
Magnetic resonance (MR)-guided high-intensity focused ultrasound has emerged as a clinical option for palliative treatment of painful bone metastases, with MR thermometry (MRT) used for treatment monitoring. In this study, the general image quality of the MRT was assessed in terms of signal-to-noise ratio (SNR) and apparent temperature variation. Also, MRT artifacts were scored for their occurrence and hampering of the treatment monitoring.
Methods
Analyses were performed on 224 MRT datasets retrieved from 13 treatments. The SNR was measured per voxel over time in magnitude images, in the target lesion and surrounding muscle, and was averaged per treatment. The standard deviation over time of the measured temperature per voxel in MRT images, in the muscle outside the heated region, was defined as the apparent temperature variation and was averaged per treatment. The scored MRT artifacts originated from the following sources: respiratory and non-respiratory time-varying field inhomogeneities, arterial ghosting, and patient motion by muscle contraction and by gross body movement. Distinction was made between lesion type, location, and procedural sedation and analgesic (PSA).
Results
The average SNR was highest in and around osteolytic lesions (21 in lesions, 27 in surrounding muscle, n = 4) and lowest in the upper body (9 in lesions, 16 in surrounding muscle, n = 4). The average apparent temperature variation was lowest in osteolytic lesions (1.2°C, n = 4) and the highest in the upper body (1.7°C, n = 4). Respiratory time-varying field inhomogeneity MRT artifacts occurred in 85% of the datasets and hampered treatment monitoring in 81%. Non-respiratory time-varying field inhomogeneities and arterial ghosting MRT artifacts were most frequent (94% and 95%) but occurred only locally. Patient motion artifacts were highly variable and occurred less in treatments of osteolytic lesions and using propofol and esketamine as PSA.
Conclusions
In this study, the general image quality of MRT was observed to be higher in osteolytic lesions and lower in the upper body. Respiratory time-varying field inhomogeneity was the most prominent MRT artifact. Patient motion occurrence varied between treatments and seemed to be related to lesion type and type of PSA. Clinicians should be aware of these observed characteristics when interpreting MRT images.
References
- Li C, Zhang W, Fan W, Huang J, Zhang F, Wu P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer. 2010;116(16):3934–42. doi:10.1002/cncr.25192.View ArticlePubMedGoogle Scholar
- Wu F, Chen WZ, Bai J, Zou JZ, Wang ZL, Zhu H, et al. Pathological changes in human malignant carcinoma treated with high-intensity focused ultrasound. Ultrasound Med Biol. 2001;27(8):1099–106.View ArticlePubMedGoogle Scholar
- Orgera G, Monfardini L, Della Vigna P, Zhang L, Bonomo G, Arnone P, et al. High-intensity focused ultrasound (HIFU) in patients with solid malignancies: evaluation of feasibility, local tumour response and clinical results. Radiol Med. 2011;116(5):734–48. doi:10.1007/s11547-011-0634-4.View ArticlePubMedGoogle Scholar
- Chen W, Zhu H, Zhang L, Li K, Su H, Jin C, et al. Primary bone malignancy: effective treatment with high-intensity focused ultrasound ablation. Radiology. 2010;255(3):967–78. doi:10.1148/radiol.10090374.View ArticlePubMedGoogle Scholar
- Leslie T, Ritchie R, Illing R, Ter Haar G, Phillips R, Middleton M, et al. \High-intensity focused ultrasound treatment of liver tumours: post-treatment MRI correlates well with intra-operative estimates of treatment volume. Br J Radiol. 2012;85(1018):1363–70. doi:10.1259/bjr/56737365.View ArticlePubMed CentralPubMedGoogle Scholar
- Kennedy JE, Wu F, ter Haar GR, Gleeson FV, Phillips RR, Middleton MR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics. 2004;42(1–9):931–5. doi:10.1016/j.ultras.2004.01.089.View ArticlePubMedGoogle Scholar
- Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, et al. Advanced hepatocellular carcinoma: treatment with high-intensity focused ultrasound ablation combined with transcatheter arterial embolization. Radiology. 2005;235(2):659–67. doi:10.1148/radiol.2352030916.View ArticlePubMedGoogle Scholar
- Zhu H, Zhou K, Zhang L, Jin C, Peng S, Yang W, et al. High intensity focused ultrasound (HIFU) therapy for local treatment of hepatocellular carcinoma: role of partial rib resection. Eur J Radiol. 2009;72(1):160–6. doi:10.1016/j.ejrad.2008.07.003.View ArticlePubMedGoogle Scholar
- Xu G, Luo G, He L, Li J, Shan H, Zhang R, et al. Follow-up of high-intensity focused ultrasound treatment for patients with hepatocellular carcinoma. Ultrasound Med Biol. 2011;37(12):1993–9. doi:10.1016/j.ultrasmedbio.2011.08.011.View ArticlePubMedGoogle Scholar
- Illing RO, Kennedy JE, Wu F, ter Haar GR, Protheroe AS, Friend PJ, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93(8):890–5. doi:10.1038/sj.bjc.6602803.View ArticlePubMed CentralPubMedGoogle Scholar
- Jung SE, Cho SH, Jang JH, Han JY. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: complications. Abdom Imaging. 2011;36(2):185–95. doi:10.1007/s00261-010-9628-2.View ArticlePubMedGoogle Scholar
- Zhang Y, Zhao J, Guo D, Zhong W, Ran L. Evaluation of short-term response of high intensity focused ultrasound ablation for primary hepatic carcinoma: utility of contrast-enhanced MRI and diffusion-weighted imaging. Eur J Radiol. 2011;79(3):347–52. doi:10.1016/j.ejrad.2010.06.039.View ArticlePubMedGoogle Scholar
- Napoli A, Anzidei M, Ciolina F, Marotta E, Cavallo Marincola B, Brachetti G, et al. MR-guided high-intensity focused ultrasound: current status of an emerging technology. Cardiovasc Intervent Radiol. 2013;36(5):1190–203. doi:10.1007/s00270-013-0592-4.View ArticlePubMedGoogle Scholar
- Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236(3):1034–40. doi:10.1148/radiol.2362041105.View ArticlePubMedGoogle Scholar
- Ritchie RW, Leslie T, Phillips R, Wu F, Illing R, ter Haar G, et al. Extracorporeal high intensity focused ultrasound for renal tumours: a 3-year follow-up. BJU Int. 2010;106(7):1004–9. doi:10.1111/j.1464-410X.2010.09289.x.View ArticlePubMedGoogle Scholar
- Hynynen K, Pomeroy O, Smith DN, Huber PE, McDannold NJ, Kettenbach J, et al. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: a feasibility study. Radiology. 2001;219(1):176–85. doi:10.1148/radiology.219.1.r01ap02176.View ArticlePubMedGoogle Scholar
- Gianfelice D, Khiat A, Amara M, Belblidia A, Boulanger Y. MR imaging-guided focused US ablation of breast cancer: histopathologic assessment of effectiveness– initial experience. Radiology. 2003;227(3):849–55. doi:10.1148/radiol.2281012163.View ArticlePubMedGoogle Scholar
- Zippel DB, Papa MZ. The use of MR imaging guided focused ultrasound in breast cancer patients; a preliminary phase one study and review. Breast Cancer (Tokyo, Japan). 2005;12(1):32–8.View ArticleGoogle Scholar
- Furusawa H, Namba K, Nakahara H, Tanaka C, Yasuda Y, Hirabara E, et al. The evolving non-surgical ablation of breast cancer: MR guided focused ultrasound (MRgFUS). Breast Cancer (Tokyo, Japan). 2007;14(1):55–8.View ArticleGoogle Scholar
- Tempany CM, Stewart EA, McDannold N, Quade BJ, Jolesz FA, Hynynen K. MR imaging-guided focused ultrasound surgery of uterine leiomyomas: a feasibility study. Radiology. 2003;226(3):897–905. doi:10.1148/radiol.2271020395.View ArticlePubMedGoogle Scholar
- Hindley J, Gedroyc WM, Regan L, Stewart E, Tempany C, Hynyen K, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: early results. AJR Am J Roentgenol. 2004;183(6):1713–9. doi:10.2214/ajr.183.6.01831713.View ArticlePubMedGoogle Scholar
- Funaki K, Fukunishi H, Sawada K. Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol. 2009;34(5):584–9. doi:10.1002/uog.7455.View ArticlePubMedGoogle Scholar
- Ikink ME, Voogt MJ, Verkooijen HM, Lohle PN, Schweitzer KJ, Franx A, et al. Mid-term clinical efficacy of a volumetric magnetic resonance-guided high-intensity focused ultrasound technique for treatment of symptomatic uterine fibroids. Eur Radiol. 2013;23(11):3054–61. doi:10.1007/s00330-013-2915-x.View ArticlePubMedGoogle Scholar
- Catane R, Beck A, Inbar Y, Rabin T, Shabshin N, Hengst S, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases–preliminary clinical experience. Ann Oncol. 2007;18(1):163–7. doi:10.1093/annonc/mdl335.View ArticlePubMedGoogle Scholar
- Gianfelice D, Gupta C, Kucharczyk W, Bret P, Havill D, Clemons M. Palliative treatment of painful bone metastases with MR imaging–guided focused ultrasound. Radiology. 2008;249(1):355–63. doi:10.1148/radiol.2491071523.View ArticlePubMedGoogle Scholar
- Liberman B, Gianfelice D, Inbar Y, Beck A, Rabin T, Shabshin N, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: a multicenter study. Ann Surg Oncol. 2009;16(1):140–6. doi:10.1245/s10434-008-0011-2.View ArticlePubMedGoogle Scholar
- Napoli A, Anzidei M, Marincola BC, Brachetti G, Ciolina F, Cartocci G, et al. Primary pain palliation and local tumor control in bone metastases treated with magnetic resonance-guided focused ultrasound. Invest Radiol. 2013;48(6):351–8. doi:10.1097/RLI.0b013e318285bbab.View ArticlePubMedGoogle Scholar
- Hurwitz MD, Ghanouni P, Kanaev SV, Iozeffi D, Gianfelice D, Fennessy FM et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: phase III trial results. J Natl Cancer Inst. 2014;106(5). doi:10.1093/jnci/dju082.Google Scholar
- Hynynen K, DeYoung D. Temperature elevation at muscle-bone interface during scanned, focused ultrasound hyperthermia. Int J Hyperthermia. 1988;4(3):267–79.View ArticlePubMedGoogle Scholar
- Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79(4):965–76. doi:10.1016/j.ijrobp.2010.11.026.View ArticlePubMedGoogle Scholar
- Huisman M, van den Bosch MA, Wijlemans JW, van Vulpen M, van der Linden YM, Verkooijen HM. Effectiveness of reirradiation for painful bone metastases: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2012;84(1):8–14. doi:10.1016/j.ijrobp.2011.10.080.View ArticlePubMedGoogle Scholar
- Mansfield P. Imaging by nuclear magnetic resonance. J Phys E Sci Instrum. 1988;21(1):18.View ArticleGoogle Scholar
- Du J, Hamilton G, Takahashi A, Bydder M, Chung CB. Ultrashort echo time spectroscopic imaging (UTESI) of cortical bone. Soc Magn Reson Med. 2007;58(5):1001–9. doi:10.1002/mrm.21397.View ArticleGoogle Scholar
- De Poorter J, De Wagter C, De Deene Y, Thomsen C, Stahlberg F, Achten E. Noninvasive MRI thermometry with the proton resonance frequency (PRF) method: in vivo results in human muscle. Soc Magn Reson Med. 1995;33(1):74–81.View ArticleGoogle Scholar
- Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K, et al. A precise and fast temperature mapping using water proton chemical shift. Soc Magn Reson Med. 1995;34(6):814–23.View ArticleGoogle Scholar
- Huisman M, Lam MK, Bartels LW, Nijenhuis RJ, Moonen CT, Knuttel FM, et al. Feasibility of volumetric MRI-guided high intensity focused ultrasound (MR-HIFU) for painful bone metastases. J Therapeutic Ultrasound. 2014;2:16. doi:10.1186/2050-5736-2-16.View ArticleGoogle Scholar
- Kohler MO, Mougenot C, Quesson B, Enholm J, Le Bail B, Laurent C, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys. 2009;36(8):3521–35.View ArticlePubMedGoogle Scholar
- Schenck JF. The role of magnetic susceptibility in magnetic resonance imaging: MRI magnetic compatibility of the first and second kinds. Med Phys. 1996;23(6):815–50.View ArticlePubMedGoogle Scholar
- Perman WH, Moran PR, Moran RA, Bernstein MA. Artifacts from pulsatile flow in MR imaging. J Comput Assist Tomogr. 1986;10(3):473–83.PubMedGoogle Scholar
- Peters NH, Bartels LW, Sprinkhuizen SM, Vincken KL, Bakker CJ. Do respiration and cardiac motion induce magnetic field fluctuations in the breast and are there implications for MR thermometry? J Magn Reson Imaging. 2009;29(3):731–5. doi:10.1002/jmri.21680.View ArticlePubMedGoogle Scholar
- Marieb EN, Koehn K. Human Anatomy and Physiology. 7th ed. San Francisco, CA: Pearson Benjamin Cunnings; 2007.Google Scholar
- Deckers R, DenisdeSenneville B, Schubert G, Merckel LG, Vaessen HHB, Vanden B, et al. Evaluation of Respiration-Induced Magnetic Field Disturbance Correction of MR Thermometry in Volunteers and in Patients for MR-HIFU Ablation of Breast Cancer: The Effects of Conscious Sedation. Milan: International Society of Magnetic Resonance in Medicine; 2014.Google Scholar
- Schmitt A, Mougenot C, Chopra R. Spatiotemporal filtering of MR-temperature artifacts arising from bowel motion during transurethral MR-HIFU. Med Phys. 2014;41(11):113302. doi:10.1118/1.4897382.View ArticlePubMedGoogle Scholar
- Staruch R, Chopra R, Hynynen K. Hyperthermia in bone generated with MR imaging-controlled focused ultrasound: control strategies and drug delivery. Radiology. 2012;263(1):117–27. doi:10.1148/radiol.12111189.PubMedGoogle Scholar
- Vinay R, KusumDevi V. Potential of targeted drug delivery system for the treatment of bone metastasis. Drug Deliv. 2014:1-9. doi:10.3109/10717544.2014.913325.Google Scholar
- Partanen A, Yarmolenko PS, Viitala A, Appanaboyina S, Haemmerich D, Ranjan A, et al. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. Int J Hyperthermia. 2012;28(4):320–36. doi:10.3109/02656736.2012.680173.View ArticlePubMedGoogle Scholar
- Hey S, Maclair G, de Senneville BD, Lepetit-Coiffe M, Berber Y, Kohler MO, et al. Online correction of respiratory-induced field disturbances for continuous MR-thermometry in the breast. Soc Magn Reson Med. 2009;61(6):1494–9. doi:10.1002/mrm.21954.View ArticleGoogle Scholar
- Wyatt CR, Soher BJ, MacFall JR. Correction of breathing-induced errors in magnetic resonance thermometry of hyperthermia using multiecho field fitting techniques. Med Phys. 2010;37(12):6300–9.View ArticlePubMed CentralPubMedGoogle Scholar
- Vigen KK, Daniel BL, Pauly JM, Butts K. Triggered, navigated, multi-baseline method for proton resonance frequency temperature mapping with respiratory motion. Soc Magn Reson Med. 2003;50(5):1003–10. doi:10.1002/mrm.10608.View ArticleGoogle Scholar
- Han M, Scott SJ, Ozhinsky E, Salgaonkar V, Larson PEZ, Diederich CJ, et al., editors. Imaging Temperature Changes in Cortical Bone using Ultrashort Echo-time MRI. Milan: International Society of Magnetic Resonance Imaging; 2014.Google Scholar
- Ramsay E, Mougenot C, Kazem M, Laetsch TW, Chopra R. Temperature-dependent MR signals in cortical bone: potential for monitoring temperature changes during high-intensity focused ultrasound treatment in bone. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2014. doi:10.1002/mrm.25492.Google Scholar
Copyright
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Gerelateerde artikelen
- HIFU - High Intensity Focused Ultrasound blijkt een effectieve behandeling voor alvleesklierkanker. HIFU gebruikt gerichte hitte om kankercellen aan te pakken en te vernietigen, en er is minimale schade aan het lichaam.
- Histotripsy, een vorm van ultra sound met gepulseerde geluidsgolven en gasbellen blijkt uitstekende, veilige en niet invasieve techniek voor eliminatie van levertumoren, daarbij gezond weefsel sparend
- UMC Utrecht start studie (i-GO studie ) met chemo via nanodeeltjes plus MR-HIFU voor borstkankerpatienten
- Zelflerende algoritmen leiden tot optimale effectiviteit van door HIFU = Ultra sound opgewekte hyperthermie bij kwaadaardige tumoren, aldus Daniel Deelen in zijn proefschrift
- HIFU - High-Intensity Focused Ultrasound is uitstekende behandeling voor niet uitgezaaide prostaatkanker, waarom blijft dit een experimentele behandeling en wordt HIFU niet vergoed vanuit basisverzekering?
- HIFU - Ultra Sound als pijnbestrijding voor in botten uitgezaaide prostaatkanker in UMC Utrecht
- Ultra sound - HIFU lijkt operatie techniek te worden voor de nabije toekomst. Het MRI-HIFU apparaat is afgelopen jaren met succes gebruikt bij vleesboom verwijdering. Hier meer informatie hoe HIFU - High Focused Ultra Sound precies werkt met foto's enz.
- Ultra Sound - opereren via geluid en verhitting - wordt ingezet bij beginnende borstkanker in het UMC - Utrecht
- Ultra Sound naast TACE geeft hoog significant betere resultaten dan alleen TACE bij levertumoren met een gemiddelde grootte van 10,4 cm. !!!!!, aldus twee gerandomiseerde studies bij totaal 200 patiënten met levertumoren.
- Ultra Sound HIFU - High Dose Focused Ultrasound bij levertumoren en niertumoren blijkt een veilig en met een beter resultaat, significant minder bijwerkingen, uit te voeren behandeling.
- Ultra sound opent tijdelijk blood - brain barriere voor gerichte chemobehandeling van tumoren in het hoofd en hersenen door nanodeeltjes.



Plaats een reactie ...
Reageer op "HIFU - Ultra Sound als pijnbestrijding voor in botten uitgezaaide prostaatkanker in UMC Utrecht"