Mocht u de informatie op onze website kanker-actueel.nl waarderen dan wilt u ons misschien ondersteunen met een donatie? Ons rekeningnummer is: RABO 37.29.31.138 t.n.v. Stichting Gezondheid Actueel in Terneuzen.
Onze IBANcode is NL79 RABO 0372 9311 38
Als donateur kunt u ook korting krijgen bij verschillende bedrijven.
En we hebben een ANBI status
10 juni 2019: Bron: JAMA en ISA-Pharma.com Met dank aan Richard die mij hier op wees.
Uit enkele kleine studies is gebleken dat wanneer kankerpatiënten die een vorm van kanker hebben die is veroorzaakt / gerelateerd aan het HPV16 - Human Papillomavirus 16 een combinatievorm van immuuntherapie krijgen met een anti-PD medicijn (Nivolumab of cemiplumab) plus een vaccin gericht op het HPV16 virus. dan reageert gemiddeld ca 33 procent met een goede reactie. En bereiken patienten die goed reageren een duurzame complete remissie of gedeeltelijke remissie. En beter dan met alleen een anti-PD medicijn.
Patienten hebben wel allemaal al een of meerdere behandelingen met chemotherapie en bestraling achter de rug maar kregen toch weer een recidief of progressie van hun ziekte.
Bv bij een studie met patiënten met mond- en keelkanker:
In deze fase II studie met nivolumab en het human papillomavirus 16 vaccine ISA101, het primaire einddoel werd al bereikt met een 33% overall response (8 of 24 patients), vergeleken met response cijfers van 16% tot 22% met alleen een anti-PD medicijn bij patiënten met vergelijkbare stadia van mond- keelkanker. Mediane overall overlevingscijfers waren met mediaan 17.5 maanden ook hoopgevend. Waarbij aangetekend dat in de behandelgroep de mediane overall overleving nog niet is bereikt omdat er nog patiënten leven en klinisch kankervrij zijn.
Vulvakanker, anaalkanker, mond- en keelkanker en baarmoederkanker en baarmoederhalskanker zijn de vormen van kanker waarin het HPV16 virus vaak een rol speelt.
Interessant in dit verband is ook deze reviewstudie over de resultaten met anti-PD medicijnen plus aanvullende vaccins bij andere vormen van kanker:
Many preclinical and clinical trials using these different strategies have been performed and we will focus here on those that have reached phase II/III clinical trials (16, 17). Some examples are provided below and in Table 1 (18–29).
Table 1
Some examples of phase II/III clinical trials testing therapeutic cancer vaccines.
| Vaccine | Cancer type | Trial phase | Trial outcomes | ClinicalTrials.gov Identifier and References |
|---|---|---|---|---|
| DC-BASED VACCINE | ||||
| Peptide-pulsed DCs | Advanced melanoma | Phase III | DC vaccination was ineffective compared to chemotherapy | Schadendorf et al. (18) |
| VACCINE USING VIRAL VECTOR | ||||
| TG4010: MVA expressing MUC-1 and IL-2 | mRCC | Phase II | Induction of an immunological response against MUC-1 has been observed and safety has been established. No clinical benefit following vaccination | Oudard et al. (19) |
| TG4010: MVA expressing MUC-1 and IL-2 | NSCLC | Phase II (TIME clinical trial) | Patients showing a MUC-1-specific response (n = 16) had an improved clinical outcome with a median overall survival (OS) of 32.1 vs. 12.7 months in non-responders (n = 6) | NCT01383148 Tosch et al. (20) |
| TroVax: MVA expressing fetal oncogene 5T4 (MVA-5T4) | Renal cancer | Phase III | No clinical benefit | NCT00397345 Amato et al. (21) |
| PROSTVAC (or PSA-TRICOM): MVA expressing PSA | mCRPC (metastatic castration-resistant prostate cancer) | Phase II | 44% reduction in death rate and an 8.5 month improvement in median OS | NCT00078585 Kantoff et al. (22) |
| TUMOR CELL-BASED VACCINE | ||||
| GVAX | Metastatic hormone-refractory prostate cancer | Phase I/II | Dose escalation study. The immunotherapy was well-tolerated. The median survival time was 35.0 months in the high-dose group, 20.0 months in the mid-dose group and 23.1 months in the low-dose group | NCT00140348 Higano et al. (23) |
| Allogenic GM-CSF secreting tumor | Pancreatic adenocarcinoma | Phase II | Vaccine combined with chemoradiation is safe. Median disease-free survival was 17.3 months and median OS was 24.8 months, which compares favorably with published data | NCT00084383 Lutz et al. (24) |
| PROTEIN/PEPTIDE-BASED VACCINE | ||||
| recMAGE-A3 protein + AS15 immunostimulant | Completely resected stage IB, II, and IIIA MAGEA3+ NSCLC | Phase III | Vaccination failed to increase the disease-free survival of surgically resected NSCLC patients | NCT00480025 Vansteenkiste et al. (25) |
| Rindopepimut with GM-CSF plus temozolomide | Newly diagnosed EGFRvIII+ glioma patients | Phase III | Rindopepimut did not increase survival in newly diagnosed glioblastoma patients | NCT01480479 Weller et al. (26) |
| IMA901: 10 different synthetic tumor-associated peptide | mRCC | Phase II | Vaccination was safe and well-tolerated. Median OS was 23.5 months for patients pretreated with cyclophosphamide | NCT00523159 Walter et al. (27) |
| Tecemotide (L-BLP25): MUC-1-derived lipopeptide | NSCLC (stage III) | Phase III | No significant difference in OS. | NCT00409188 Butts et al. (28) |
| Multiepitope vaccine composed of tyrosinase, gp100 and MART-1 peptides | High-risk resected melanoma | Phase III | No RFS or OS improvement in vaccinated patients | NCT01989572 Lawson et al. (29) |
Verder over het HPV vaccin:
Het zijn nog wel kleine aantallen patiënten die aan deze studies hebben meegedaan maar inmiddels zijn enkele fase II studies geopend met grotere aantallen patiënten. Wel placebo gecontroleerd in die zin dat de ene helft de immuunbehandeling met het vaccin krijgt en de andere groep de standaard behandeling uit de richtlijnen.
Hier volgen respectievelijk twee studieresultaten en een studieprotocol. De Nederlandse Sonja Visscher werkend voor de producent kan om inlichtingen worden gevraagd voor andere lopende studies met dit vaccin:
| Contact: Sonja Visscher | +31713322310 | Visscher@isa-pharma.com |
Hier het studieprotocol van A Randomized Phase 2 Study of Cemiplimab ± ISA101b in HPV16-Positive OPC
Op basis van deze resultaten, gehaald van de website van de producent van dit vaccin:
ISA101 has completed a Phase 2 trial in vulvar intra-epithelial neoplasia, establishing clinical proof-of-concept. In cervical cancer, ISA101 has successfully completed a company-sponsored Phase 1/2 trial and has entered into further clinical development in collaboration with Regeneron. The alliance aims to develop and advance ISA101 in combination with cemiplimab (Libtayo®), a PD-1 (programmed cell death protein 1) antibody currently under review by EMA and initially approved by the U.S. Food and Drug Administration in September 2018 under the brand name Libtayo® as monotherapy for patients with advanced cutaneous squamous cell carcinoma.
Wat is het ISA101 vaccin eigenlijk?
ISA101 consists of 12 synthetic long peptides (25 to 35 amino acids long) derived from the E6 and E7 oncogenic proteins of the HPV 16 virus, a strain responsible for over 50% of human cervical cancers and cervical intra-epithelial neoplasias, more than 85% of HPV-positive head and neck cancers, and similar substantial percentages of other premalignant and malignant HPV-induced lesions (such as VIN, vulvar cancer, AIN and anal cancer). It is currently administered either subcutaneously or intradermally.
Although most HPV16 infections cause no symptoms, are self-limited and cleared by a natural immune response, in some cases the virus becomes integrated into the DNA of cells, causing persistent infections that may lead to precancerous lesions and cancer.
Several prophylactic vaccines preventing HPV infections caused by a number of prevalent HPV types (2, 4, or 9 valent) are currently on the market. However, these vaccines are limited to preventing HPV infections and cannot cure established viral infections and associated lesions nor cancer once HPV is incorporated into the genomic DNA.
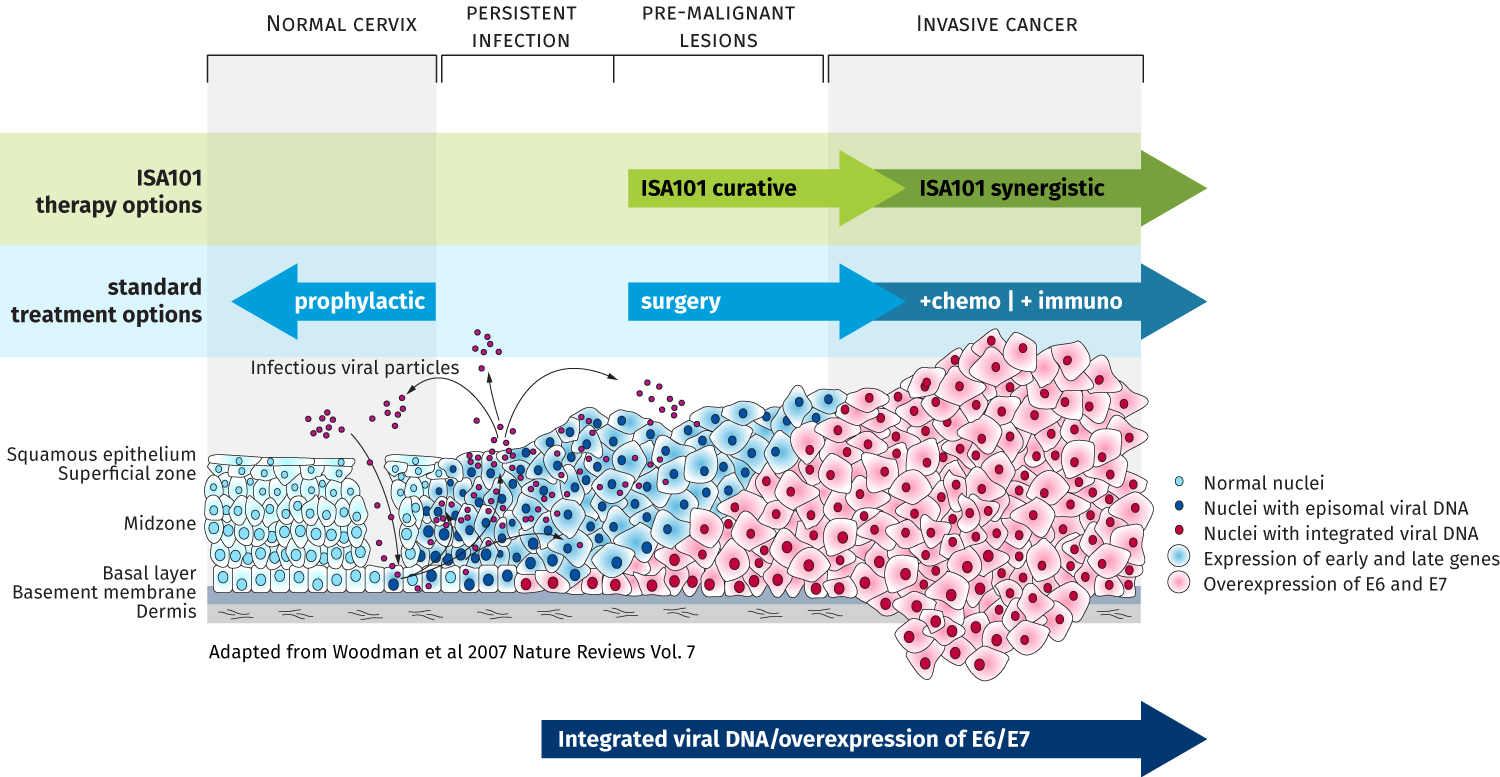
With its ISA101 immunotherapeutic, ISA Pharmaceuticals is targeting the gap between prophylactic vaccines and standard-of-care cancer treatments. The combined total market opportunity addressed by an immunotherapeutic against HPV16 amounts to a multi-billion-dollar market annually.
Een andere studie werd in januari 2019 in JAMA gepubliceerd:
Hier het abstract en studieprotocol van deze studie:
Gerelateerde artikelen



Plaats een reactie ...
Reageer op "Baarmoederhalskanker: Immuuntherapie met anti-PD medicijn Cemiplimab plus het vaccin ISA101b gericht op het HPV16 virus of met nivolumab geeft veelbelovende resultaten voor vormen van kanker die HPV gerelateerd zijn"