Zie ook deze lijst specifiek gerelateerd aan radiotherapie - bestraling:
https://kanker-actueel.nl/NL/studiepublicaties-van-niet-toxische-middelen-en-behandelingen-uit-literatuurlijst-van-arts-bioloog-drs-engelbert-valstar-naast-radiotherapie-bestraling-bij-alle-vormen-van-kanker.html
7 januari 2017
N.a.v. reactie van Heleen onderaan dit artikel heb ik wat informatie erbij gezocht, ook omdat de bestralingstechnieken veel verbeterd zijn de laatste tien jaar is het risico op hartfalen zoals in onderstaand artikel wel sterk verminderd. Hoewel bestraling van borstgebied zeker niet helemaal zonder risico is, zeker niet voor jonge mensen. Maar ook worden tegenwoordig effectieve hartbeschermingsmiddelen gebruikt, zoals deze (tekst gaat onder foto verder):
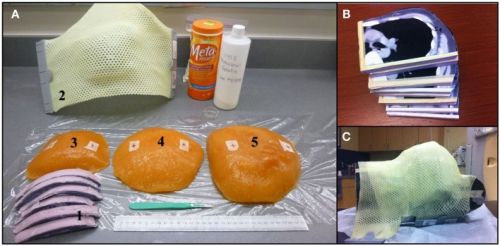
Bron foto: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4205812/
Hier enkele belangrijke studies in relatie tot dit onderwerp en klik op de omschrijving om het studierapport in te zien: Patterns and Correlates of Adjuvant Radiotherapy Receipt After Lumpectomy and After Mastectomy for Breast Cancer
Met conclusie:
The results suggest that we have largely achieved success in the appropriate use of RT after BCS in metropolitan areas like those we studied, but more attention needs to be paid to the use of RT after mastectomy. We found that surgeon participation in the RT decision was a powerful correlate of use. This underscores the need to focus physician attention on potential gaps in treatment delivery. We also found that patient concerns about radiation were negatively associated with RT use. This is important because patients with these concerns may choose mastectomy with the intention of avoiding radiation, resulting in a higher prevalence of these concerns in patients undergoing mastectomy than undergoing BCS.43 Thus, it is important to consider these concerns when informing these patients about RT. Our findings suggest that initiatives to ensure that surgeons are informed about the role of RT after mastectomy, to encourage provider participation in the postmastectomy RT decision, and to improve patient education in this setting, would further optimize care for patients with breast cancer.
Tekst gaat onder foto verder
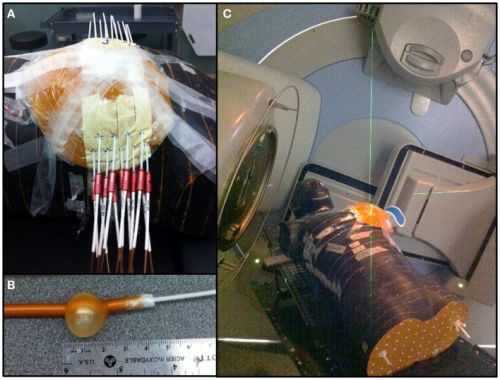
Foto van hartbeschermingkomt uit de volgende studie:
Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300 000 women in US SEER cancer registries
met als conclusie:
US breast cancer radiotherapy regimens of the 1970s and early 1980s appreciably increased mortality from heart disease and lung cancer 10–20 years afterwards with, as yet, little direct evidence on the hazards after more than 20 years. Since the early 1980s, improvements in radiotherapy planning should have reduced such risks, but the long-term hazards in the general populations of various countries still need to be monitored directly.
Andere recente studie met uitstekende referentielijst, zie ook onderaan dit artikel:
Measurement of mean cardiac dose for various breast irradiation techniques and corresponding risk of major cardiovascular event.
Conclusie uit deze studie:
WBI lead to the highest mean heart dose (2.99 Gy) compared to 3D-CRT APBI (0.51 Gy), multicatheter (1.58 Gy), and balloon HDR (2.17 Gy) for a medially located tumor. This translated into long-term coronary event increases of 22, 3.8, 11.7, and 16% respectively. The sensitivity analysis showed that the tumor location had almost no effect on the mean heart dose for 3D-CRT APBI and a minimal impact for HDR APBI. In case of WBI large breast size and set-up errors lead to sharp increases of the mean heart dose. Its value reached 10.79 Gy for women with large breast and a set-up error of 1.5 cm. Such a high value could increase the risk of having long-term coronary events by 80%. Comparison among different irradiation techniques demonstrates that 3D-CRT APBI appears to be the safest one with less probability of having cardiovascular events in the future. A sensitivity analysis showed that WBI is the most challenging technique for patients with large breasts or when significant set-up errors are anticipated. In those cases, additional heart shielding techniques are required.
Ook de volgende studie geeft uitstekende informatie:
Factors associated with guideline-concordant use of radiation therapy after mastectomy in the National Comprehensive Cancer Network
Conclusie:
In summary, we found that rates of concordance with definitive guidelines for PMRT were high. Appropriate use of PMRT among the high-risk cohort was the rule, while overuse of PMRT among women with low risk disease occurred infrequently. The only factor associated with underuse was non-receipt of chemotherapy, suggesting that care may appropriately reflect patient preferences. Although overuse was rare, it appeared to be driven by provider biases, and in particular, a tendency to treat women with higher risk disease more aggressively despite the absence of consensus opinion supporting this approach. Finally, we found considerable variability in the use of PMRT among women at moderate risk of recurrence, highlighting the need for focused clinical research in this group.
In its report “Ensuring Quality Cancer Care,” the National Cancer Policy Board5 recommended the use of radiation therapy following breast-conserving surgery as a good process indicator to study the quality of cancer care. Indeed, prior studies have largely focused on the use of radiation therapy after breast-conserving surgery as such a measure. Radiation therapy after mastectomy may also be an important quality indicator and national target for intervention, given its significant survival impact and the large number of lives at stake. The challenge will be to develop a quality monitoring system that includes sufficient clinical detail to identify women most likely to benefit from PMRT.
Zie verder studies in referentielijst onderaan artikel:
20 augustus 2007 Bron: Yahoonews
Vrouwen waarvan de linkerborst is bestraald na een operatie blijken een veel groter risico op hartklachten te hebben als zij de kanker overleven dan vrouwen waarvan de rechterborst is bestraald. Er bleek gemeten over mediaan 12 jaar een verschil te zijn voor de linkerborst t.o.v. de rechterborst van 51% en is hoog significant. (8% om 59%). Dit blijkt uit een gerandomiseerde studie bij 961 vrouwen en de studie is gepubliceerd in Clinical Oncology.
Left-sided breast cancer radiation ups heart risk
http://news.yahoo.com/s/nm/20070809/hl_nm/breast_radiation_dc
By David Douglas Thu Aug 9, 6:40 PM ET
NEW YORK (Reuters Health) - Women with early-stage cancer of the left breast who are treated with radiation as a component of breast-sparing treatment, have an increased risk of developing radiation-related coronary damage, researchers report.
Nevertheless, "the benefits of radiation therapy for breast cancer still clearly outweigh the risks," Dr. Candace R. Correa told Reuters Health. "However," she added, "there may still be room for improvement in radiation techniques," when radiation is applied to the breast on the same side as the heart.
Correa, at the University of Michigan, Ann Arbor, and colleagues examined the medical records of 961 stage I-II breast cancer patients to look into this issue of radiation damage to the heart's arteries.
At the time they were diagnosed, women with left-sided and those with right-sided breast cancers had the same likelihood of developing coronary artery disease. At an average of 12 years after radiation treatment, 46 of the 485 left-sided women and 36 of 476 in the right-sided group had needed cardiac stress testing, the team reports in the Journal of Clinical Oncology.
The results showed that among those tested, 59 percent in the left-sided group had abnormalities, significantly more than the 8 percent in the right-sided group.
"Careful monitoring and long-term follow-up to assess these risks ... is important," Correa stressed.
For patients, she added, "it is most prudent to optimize their cardiovascular health by living a healthy lifestyle and speaking with their doctors about risk reduction tools and interventions that may be appropriate for their situation."
SOURCE: Journal of Clinical Oncology, July 20, 2007.
References
1.
Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med (2013) 368:987–98.10.1056/NEJMoa1209825 [PubMed] [Cross Ref]2.
DeSantis C, Ma J, Bryan L, Jemal A.. Breast cancer statistics, 2013. CA Cancer J Clin (2014) 64:52–52.10.3322/caac.21203 [PubMed] [Cross Ref]3.
Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD.. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol (2012) 36:237–48.10.1016/j.canep.2012.02.007 [PubMed] [Cross Ref]4.
Gradishar WJ, Anderson BO, Blair SL, Burstein HJ, Cyr A, Elias AD, et al. Breast cancer version 3.214. J Natl Compr Canc Netw (2014) 12:542–90. [PubMed]5.
START Trialist Group. Betzen SM, Agrawal RK, Air EG, Barret JM, Barret-Lee PJ, et al. The UK standardisation of breast radiotherapy (START) trial A of radiotherapy hypofractionation for the treatment of early breast cancer: a randomized trial. Lancet Oncol (2008) 9:331–41.10.1016/S1470-2045(08)70077-9 [PMC free article] [PubMed] [Cross Ref]6.
START Trialist Group. Betzen SM, Agrawal RK, Air EG, Barret JM, Barret-Lee PJ, et al. The UK standardisation of breast radiotherapy (START) trial B of radiotherapy hypofractionation for the treatment of early breast cancer: a randomized trial. Lancet Oncol (2008) 371:1098–107.10.1016/S0140-6736(08)60348-7 [PMC free article] [PubMed] [Cross Ref]7.
Whelan TJ, Pignol JP, Levine MN, Julian JA, Mackenzie R, Parpia S, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med (2010) 362:513–20.10.1056/NEJMoa0906260 [PubMed] [Cross Ref]8.
Smith BD, Arthur DW, Buchholtz TA, Haffty BG, Hahn CA, Hardenbergh PH, et al. Accelerated partial breast irradiation consensus statement from the American society for radiation oncology (ASTRO). Int J Radiat Oncol Biol Phys (2009) 74:987–1001.10.1016/j.ijrobp.2009.02.031 [PubMed] [Cross Ref]9.
Polgar C, Van Limbergen E, Potter R, Kovacs G, Polo A, Lyczek J, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the groupe Européen de curiethérapie – European society for therapeutic radiology and oncology (GEC-ESTRO) breast cancer working group based on clinical evidence. Radiother Oncol (2010) 94:264–73.10.1016/j.radonc.2010.01.014 [PubMed] [Cross Ref]10.
Njeh CF, Saunders M, Langton C.. Accelerated partial breast irradiation (APBI): a review of available techniques. Radiat Oncol (2010) 5:90.10.1186/1748-717X-5-90 [PMC free article] [PubMed] [Cross Ref]11.
Pignol JP, Rakovitch E, Keller BM, Sankreacha R, Chartier C.. Tolerance and acceptance results of a palladium-103 permanent breast seed implant phase I/II study. Int J Radiat Oncol Biol Phys (2009) 73:1482–8.10.1016/j.ijrobp.2008.06.1945 [PubMed] [Cross Ref]12.
Shah C, Badiyan S, Ben Wilkinson J, Vicini F, Beitsch P, Keisch M, et al. Treatment efficacy with accelerated partial breast irradiation (APBI): final analysis of the American society of breast surgeons mammosite breast brachytherapy registry trial. Ann Surg Oncol (2013) 20:3279–85.10.1245/s10434-013-3158-4 [PubMed] [Cross Ref]13.
Vaidya JS, Wenz F, Bulsara M, Tobias JS, Joseph DJ, Keshtgar M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet (2014) 383:603–13.10.1016/S0140-6736(13)61950-9 [PubMed] [Cross Ref]14.
Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER Cancer Statistics Review, 1975-2011. Bethesda, MD: National Cancer Institute; (2014). Available from: http://seer.cancer.gov/csr/1975_2011/15.
Cuzick J, Stewart H, Rutqvist L, Houghton J, Edwards R, Redmond C, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol (1994) 12:447–53. [PubMed]16.
Tjessem KH, Johansen S, Malinen E, Reinertsen KV, Danielsen T, Fosså SD, et al. Long-term cardiac mortality after hypofractionated radiation therapy in breast cancer. Int J Radiat Oncol Biol Phys (2013) 87:337–43.10.1016/j.ijrobp.2013.05.038 [PubMed] [Cross Ref]17.
Paszat LF, Mackillop WJ, Groome PA, Boyd C, Schulze K, Holowaty E.. Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in the surveillance, epidemiology, and end-results cancer registries. J Clin Oncol (1998) 16:2625–31. [PubMed]18.
Darby SC, McGale P, Taylor CW, Peto R.. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol (2005) 6:557–65.10.1016/S1470-2045(05)70251-5 [PubMed] [Cross Ref]19.
Lettmaier S, Kreppner S, Lotter M, Walser M, Ott OJ, Fietkau R, et al. Radiation exposure of the heart, lung and skin by radiation therapy for breast cancer: a dosimetric comparison between partial breast irradiation using multicatheter brachytherapy and whole breast teletherapy. Radiother Oncol (2011) 100:189–94.10.1016/j.radonc.2010.07.011 [PubMed] [Cross Ref]20.
Garza R, Albuquerque K, Sethi A.. Lung and cardiac tissue doses in left breast cancer patients treated with single-source breast brachytherapy compared to external beam tangent fields. Brachytherapy (2006) 5:235–8.10.1016/j.brachy.2006.08.001 [PubMed] [Cross Ref]21.
Weed DW, Edmunson GK, Vicini FA, Chen PY, Martinez AA.. Accelerated partial breast irradiation: a dosimetric comparison of three different techniques. Brachytherapy (2005) 4:121–9.10.1016/j.brachy.2004.12.005 [PubMed] [Cross Ref]22.
Major T, Niehoff P, Kovacs G, Fodor J, Polgar C.. Dosimetric comparisons between high dose rate interstitial mamosite balloon brachytherapy for breast cancer. Radiother Oncol (2006) 79:321–8.10.1016/j.radonc.2006.05.005 [PubMed] [Cross Ref]23.
Khan AJ, Kirk MC, Mehta PS, Seif NS, Griem KL, Bernard DA, et al. A dosimetric comparison of three-dimensional conformal, intensity modulated radiation therapy, and mamosite partial-breast irradiation. Brachytherapy (2006) 5:183–8.10.1016/j.brachy.2006.06.001 [PubMed] [Cross Ref]24.
Stewart AJ, O’Farrell DA, Cormack RA, Hansen JL, Khan AJ, Mutyala S, et al. Dose volume histogram analysis of normal structures associated with accelerated partial breast irradiation delivered by high dose rate brachytherapy and comparison with whole breast external beam radiotherapy. Radiat Oncol (2008) 19:3–39.10.1186/1748-717X-3-39 [PMC free article] [PubMed] [Cross Ref]25.
Valakh V, Kim Y, Werts ED, Trombetta MG.. A comprehensive analysis of cardiac dose in balloon-based high-dose-rate brachytherapy for left-sided breast cancer. Int J Radiat Oncol Biol Phys (2012) 82:1698–705.10.1016/j.ijrobp.2011.02.058 [PubMed] [Cross Ref]26.
Taylor ML, Kron T.. Consideration of the radiation dose delivered away from the treatment field to patients in radiotherapy. Phys Med Biol (2011) 36:59–71.10.4103/0971-6203.79686 [PMC free article] [PubMed] [Cross Ref]27.
Howell RM, Scarboro SB, Kry SF, Yaldo DZ.. Accuracy of out-of-field dose calculations by a commercial treatment planning system. Phys Med Biol (2010) 55:6999–7008.10.1088/0031-9155/55/23/S03 [PMC free article] [PubMed] [Cross Ref]28.
Woo TC, Pignol JP, Rakovitch E, Vu T, Hicks D, O’Brien P, et al. Body radiation exposure in breast cancer radiotherapy: impact of breast IMRT and virtual wedge compensation techniques. Int J Radiat Oncol Biol Phys (2006) 65:52–8.10.1016/j.ijrobp.2005.11.023 [PubMed] [Cross Ref]29.
NSABP B-39, RTOG 0413: a randomized phase III study of conventional whole breast irradiation versus partial breast irradiation for women with stage 0, I, or II breast cancer. Clin Adv Hematol Oncol (2006) 4:719–21. [PubMed]30.
Pignol JP, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckham W, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol (2008) 26:2085–92.10.1200/JCO.2007.15.2488 [PubMed] [Cross Ref]31.
Olivotto IA, Whelan TJ, Parpia S, Kim DH, Berrang T, Truong PT, et al. Interim cosmetic and toxicity results from RAPID: a randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol (2013) 31:4038–45.10.1200/JCO.2013.50.5511 [PubMed] [Cross Ref]32.
Pierquin B, Dutreix A, Paine CH, Chassagne D, Marinello G, Ash D.. The Paris system in interstitial radiation therapy. Acta Radiol Oncol Radiat Phys Biol (1978) 17:33–48.10.3109/02841867809127689 [PubMed] [Cross Ref]33.
Lessard E, Pouliot J.. Inverse planning anatomy-based dose optimization for HDR-brachytherapy of the prostate using fast simulated annealing algorithm and dedicated objective function. Med Phys (2001) 28:773–9.10.1118/1.1368127 [PubMed] [Cross Ref]34.
Taylor CW, McGale P, Povall JM, Thomas E, Kumar S, Dodwell D, et al. Estimating cardiac exposure from breast cancer radiotherapy in clinical practice. Int J Radiat Oncol Biol Phys (2009) 73:1061–8.10.1016/j.ijrobp.2008.05.066 [PubMed] [Cross Ref]35.
Goody RB, O’Hare J, McKenna K, Dearey L, Robinson J, Bell P, et al. Unintended cardiac irradiation during left-sided breast cancer radiotherapy. Br J Radiol (2013) 86:20120434.10.1259/bjr.20120434 [PMC free article] [PubMed] [Cross Ref]36.
Pignol JP, Keller BM, Ravi A.. Doses to internal organs for various breast radiation techniques-implications on the risk of secondary cancers and cardiomyopathy. Radiat Oncol (2011) 6:5.10.1186/1748-717X-6-5 [PMC free article] [PubMed] [Cross Ref]37.
Hiatt JR, Evans SB, Price LL, Cardarelli GA, Dipetrillo TA, Wazer DE.. Dose-modeling study to compare external beam techniques from protocol NSABP B-39/RTOG 0413 for patients with highly unfavorable cardiac anatomy. Int J Radiat Oncol Biol Phys (2006) 65(5):1368–74.10.1016/j.ijrobp.2006.03.060 [PubMed] [Cross Ref]38.
Correa CR, Litt HI, Hwang WT, Ferrari VA, Solin LJ, Harris EE.. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol (2007) 25:3031–7.10.1200/JCO.2006.08.6595 [PubMed] [Cross Ref]39.
Shah C, Badiyan S, Berry S, Khan AJ, Goyal S, Schulte K, et al. Cardiac dose sparing and avoidance techniques in breast cancer radiotherapy. Radiother Oncol (2014) 112(1):9–16.10.1016/j.radonc.2014.04.009 [PubMed] [Cross Ref]40.
Swanson T, Grills IS, Ye H, Entwistle A, Teahan M, Letts N, et al. Six-year experience routinely using moderate deep inspiration breath-hold for the reduction of cardiac dose in left-sided breast irradiation for patients with early-stage or locally advanced breast cancer. Am J Clin Oncol (2013) 36:24–30.10.1097/COC.0b013e31823fe481 [PMC free article] [PubMed] [Cross Ref]41.
Jimenez RB, Goma C, Nyamwanda J, Kooy HM, Halabi T, Napolitano BN, et al. Intensity modulated proton therapy for postmastectomy radiation of bilateral implant reconstructed breasts: a treatment planning study. Radiother Oncol (2013) 107:213–7.10.1016/j.radonc.2013.03.028 [PubMed] [Cross Ref]42.
Zauls AJ, Watkins JM, Wahlquist AE, Brackett NC, III, Aguero EG, Baker MK, et al. Outcomes in women treated with mammosite brachytherapy or whole breast irradiation stratified by ASTRO accelerated partial breast irradiation consensus statement groups. Int J Radiat Oncol Biol Phys (2012) 82:21–9.10.1016/j.ijrobp.2010.08.034 [PubMed] [Cross Ref]43.
Park SS, Grills IS, Chen PY, Kestin LL, Ghilezan MI, Wallace M, et al. Accelerated partial breast irradiation for pure ductal carcinoma in situ. Int J Radiat Oncol Biol Phys (2011) 81:403–8.10.1016/j.ijrobp.2010.05.030 [PubMed] [Cross Ref]44.
Smith GL, Xu Y, Buchholz TA, Giordano SH, Jiang J, Shih YC, et al. Association between treatment with brachytherapy vs whole-breast irradiation and subsequent mastectomy, complications, and survival among older women with invasive breast cancer. JAMA (2012) 307:1827–37.10.1001/jama.2012.3481 [PMC free article] [PubMed] [Cross Ref]
Articles from Frontiers in Oncology are provided here courtesy of Frontiers Media SA
Another references list:
REFERENCES
1.
Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. [PubMed]2.
Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. [PubMed]3.
Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. [PubMed]4.
Carlson RW, Edge SB, Theriault RL. NCCN: Breast cancer. Cancer Control. 2001;8:54–61. [PubMed]5. Hewitt M, Simone JV, editors. National Cancer Policy Board: Ensuring Quality Cancer Care. Washington D. C.: National Academy Press; 1999.
6.
O'Connor AM, Mulley AG, Jr., Wennberg JE. Standard consultations are not enough to ensure decision quality regarding preference-sensitive options. J Natl Cancer Inst. 2003;95:570–571. [PubMed]7.
Wennberg JE. Unwarranted variations in healthcare delivery: implications for academic medical centres. BMJ. 2002;325:961–964. [PMC free article] [PubMed]8.
Farrow DC, Hunt WC, Samet JM. Geographic variation in the treatment of localized breast cancer. N Engl J Med. 1992;326:1097–1101. [PubMed]9.
Nattinger AB, Gottlieb MS, Veum J, et al. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992;326:1102–1107. [PubMed]10.
Riley GF, Potosky AL, Klabunde CN, et al. Stage at diagnosis and treatment patterns among older women with breast cancer: an HMO and fee-for-service comparison. JAMA. 1999;281:720–726. [PubMed]11.
Hillner BE, Penberthy L, Desch CE, et al. Variation in staging and treatment of local and regional breast cancer in the elderly. Breast Cancer Res Treat. 1996;40:75–86. [PubMed]12.
Lazovich DA, White E, Thomas DB, et al. Underutilization of breast-conserving surgery and radiation therapy among women with stage I or II breast cancer. JAMA. 1991;266:3433–3438. [PubMed]13.
Guadagnoli E, Shapiro CL, Weeks JC, et al. The quality of care for treatment of early stage breast carcinoma: is it consistent with national guidelines? Cancer. 1998;83:302–309. [PubMed]14.
Hillner BE, McDonald MK, Penberthy L, et al. Measuring standards of care for early breast cancer in an insured population. J Clin Oncol. 1997;15:1401–1408. [PubMed]15.
Lazovich D, Solomon CC, Thomas DB, et al. Breast conservation therapy in the United States following the 1990 National Institutes of Health Consensus Development Conference on the treatment of patients with early stage invasive breast carcinoma. Cancer. 1999;86:628–637. [PubMed]16.
Morrow M, White J, Moughan J, et al. Factors predicting the use of breast-conserving therapy in stage I and II breast carcinoma. J Clin Oncol. 2001;19:2254–2262. [PubMed]17.
Weeks J. Outcomes assessment in the NCCN: 1998 update. National Comprehensive Cancer Network. Oncology (Huntingt) 1999;13:69–71. [PubMed]18.
Weeks JC. Outcomes assessment in the NCCN. Oncology (Huntingt) 1997;11:137–140. [PubMed]19.
Niland JC. NCCN Internet-based data system for the conduct of outcomes research. Oncology (Huntingt) 1998;12:142–146. [PubMed]20.
Niland JC. NCCN outcomes research database: data collection via the Internet. Oncology (Huntingt) 2000;14:100–103. [PubMed]21.
Edge SB, Niland JC, Bookman MA, et al. Emergence of sentinel node biopsy in breast cancer as standard-of-care in academic comprehensive cancer centers. J Natl Cancer Inst. 2003;95:1514–1521. [PubMed]22. American Joint Committee on Cancer: AJCC Cancer Staging Manual. ed Fifth. Philadelphia: Lippincott Williams and Wilkins; 1997. pp. 171–181.
23.
Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. [PubMed]24.
Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudnal studies. J Chronic Dis. 1987;40:373–383. [PubMed]25. American Hospital Association: Hospital Statistics. ed 2000. Chicago (IL): 2001.
26.
Tran NV, Chang DW, Gupta A, et al. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2001;108:78–82. [PubMed]27.
Athas WF, Adams-Cameron M, Hunt WC, et al. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst. 2000;92:269–271. [PubMed]28.
Nattinger AB, Kneusel RT, Hoffmann RG, et al. Relationship of distance from a radiotherapy facility and initial breast cancer treatment. J Natl Cancer Inst. 2001;93:1344–1346. [PubMed]
borstkanker, bestraling, radiotherapie, risico's op hartfalen, hartbescherming
Gerelateerde artikelen





Plaats een reactie ...
1 Reactie op "Vrouwen waarvan de linkerborst is bestraald hebben significant meer risicio op hartklachten later in hun leven dan de vrouwen waarvan de rechterborst is bestraald."