Wanneer bij mensen met schildklierknobbeltjes Bethesda III of IV vooraf aan de geplande operatie een nauwkeuriger diagnose werd gedaan met een FDG-PET/CT-scan dan bleek 42 procent van de operaties niet nodig te zijn omdat de knobbeltjes goedaardig bleken. Hiermee werd dus het verschil tussen goedaardige en kwaadaardige knobbeltjes aangetoond met alle gevolgen vandien voor de patiënt. Zij kregen dus veel meer zekerheid welke behandeling nodig was.
Het primaire doel van de Nederlandse studie was het nauwkeurig verminderen van ongunstige patiëntenzorg, d.w.z. het vermijden van diagnostische chirurgie voor goedaardige knobbeltjes en het vermijden van surveillance voor kwaadaardige en borderline knobbeltjes die een chirurgische ingreep vereisen.
Secundaire doelstellingen waren het bepalen van de impact van FDG-PET/CT-gestuurd management op het aantal chirurgische complicaties, HRQoL - kwaliteit van leven, maatschappelijke kosten en gevolgen van incidentele PET/CT-bevindingen en het beoordelen van de implementeerbaarheid van FDG-PET /CT.
Vertaalde resultaten uit het abstract:
- Patiëntenbehandeling was niet gunstig bij 42% (38/91 [95% betrouwbaarheidsinterval , 32-53%]) van de patiënten in de FDG-PET/CT-aangedreven groep, vergeleken met 83% (34/ 41 [95% BI, 68-93%]) in de groep voor diagnostische chirurgie (p < 0,001).
- FDG-PET/CT-gestuurde behandeling vermeden 40% (25/63 [95% BI, 28–53%]) diagnostische operaties voor goedaardige knobbeltjes: 48% (23/48 [95% BI, 33–63%] ]) in niet-Hürthle-cellen en 13% (2/15 [95% BI, 2-40%]) in Hürthle-celknobbeltjes (p = 0.02).
- Er werden geen kwaadaardige of borderline-tumoren waargenomen bij patiënten onder toezicht.
- Gevoeligheid, specificiteit, negatief en positief voorspellende waarde en goedaardige call rate (95% BI) van FDG-PET/CT waren 94,1% (80,3-99,3%), 39,8% (30,0-50,2%), 95,1% ( 83,5-99,4%), 35,2% (25,4-45,9%) en 31,1% (23,3-39,7%), respectievelijk.
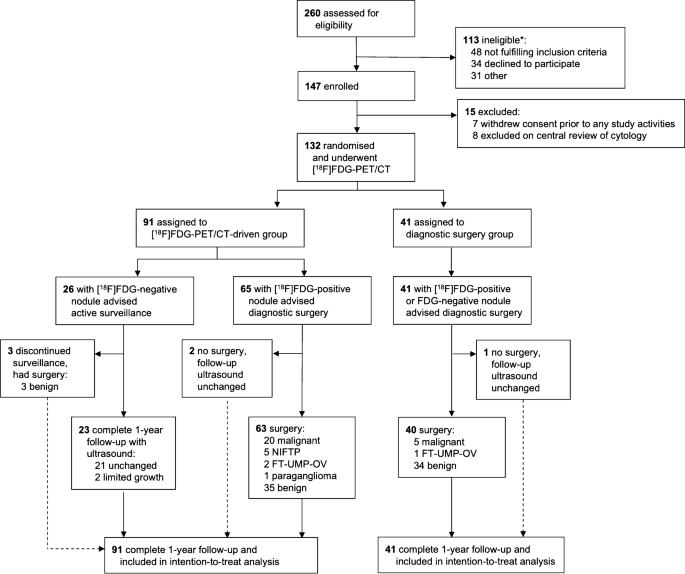
Trial profile. The dashed line indicates the patients who deviated from the treatment advise per protocol. NIFTP, non-invasive follicular thyroid neoplasm with papillary-like nuclear features. FT-UMP-OV, follicular tumour of uncertain malignant potential, Hürthle cell type. *: a specification of reasons for ineligibility is provided in Supplementary Table 2
Conclusie:
Een FDG-PET/CT-gestuurde diagnostische verbetering van onbepaalde schildklierknobbeltjes leidt tot praktijkverandering van management, is nauwkeurig en oncologisch veilig, waardoor nutteloze operaties met 40% kunnen worden verminderd. Voor een optimale therapeutische opbrengst zou de toepassing van de FDG-PET/CT kunnen worden beperkt tot niet-Hürthle-celknobbeltjes.
Het volledige studierapport is gratis in te zien of te downloaden. Klik daarvoor op de titel van het abstract:
- Original Article
- Open Access
- Published:
[18F]FDG-PET/CT to prevent futile surgery in indeterminate thyroid nodules: a blinded, randomised controlled multicentre trial
European Journal of Nuclear Medicine and Molecular Imaging (2022)
Abstract
Purpose
To assess the impact of an [18F]FDG-PET/CT-driven diagnostic workup to rule out malignancy, avoid futile diagnostic surgeries, and improve patient outcomes in thyroid nodules with indeterminate cytology.
Methods
In this double-blinded, randomised controlled multicentre trial, 132 adult euthyroid patients with scheduled diagnostic surgery for a Bethesda III or IV thyroid nodule underwent [18F]FDG-PET/CT and were randomised to an [18F]FDG-PET/CT-driven or diagnostic surgery group. In the [18F]FDG-PET/CT-driven group, management was based on the [18F]FDG-PET/CT result: when the index nodule was visually [18F]FDG-positive, diagnostic surgery was advised; when [18F]FDG-negative, active surveillance was recommended. The nodule was presumed benign when it remained unchanged on ultrasound surveillance. In the diagnostic surgery group, all patients were advised to proceed to the scheduled surgery, according to current guidelines. The primary outcome was the fraction of unbeneficial patient management in one year, i.e., diagnostic surgery for benign nodules and active surveillance for malignant/borderline nodules. Intention-to-treat analysis was performed. Subgroup analyses were performed for non-Hürthle cell and Hürthle cell nodules.
Results
Patient management was unbeneficial in 42% (38/91 [95% confidence interval , 32–53%]) of patients in the [18F]FDG-PET/CT-driven group, as compared to 83% (34/41 [95% CI, 68–93%]) in the diagnostic surgery group (p < 0.001). [18F]FDG-PET/CT-driven management avoided 40% (25/63 [95% CI, 28–53%]) diagnostic surgeries for benign nodules: 48% (23/48 [95% CI, 33–63%]) in non-Hürthle cell and 13% (2/15 [95% CI, 2–40%]) in Hürthle cell nodules (p = 0.02). No malignant or borderline tumours were observed in patients under surveillance. Sensitivity, specificity, negative and positive predictive value, and benign call rate (95% CI) of [18F]FDG-PET/CT were 94.1% (80.3–99.3%), 39.8% (30.0–50.2%), 95.1% (83.5–99.4%), 35.2% (25.4–45.9%), and 31.1% (23.3–39.7%), respectively.
Conclusion
An [18F]FDG-PET/CT-driven diagnostic workup of indeterminate thyroid nodules leads to practice changing management, accurately and oncologically safely reducing futile surgeries by 40%. For optimal therapeutic yield, application should be limited to non-Hürthle cell nodules.
Trial registration number
This trial is registered with ClinicalTrials.gov: NCT02208544 (5 August 2014), https://clinicaltrials.gov/ct2/show/NCT02208544.
References
-
National Cancer Institute. Surveillance, epidemiology, and end results (SEER) program. CancerStat Facts: Thyroid Cancer. Bethesda, MD: National Cancer Institute, DCCPS, Surveillance Research Program; 2020.
-
Durante C, Grani G, Lamartina L, Filetti S, Mandel SJ, Cooper DS. The diagnosis and management of thyroid nodules: a review. JAMA. 2018;319:914–24. https://doi.org/10.1001/jama.2018.0898.
-
Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27:1341–6. https://doi.org/10.1089/thy.2017.0500.
-
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. https://doi.org/10.1089/thy.2015.0020.
-
Vriens D, de Wilt JH, van der Wilt GJ, Netea-Maier RT, Oyen WJ, de Geus-Oei LF. The role of -2-fluoro-2-deoxy-d-glucose-positron emission tomography in thyroid nodules with indeterminate fine-needle aspiration biopsy: systematic review and meta-analysis of the literature. Cancer. 2011;117:4582–94. https://doi.org/10.1002/cncr.26085.
-
Rosato L, Avenia N, Bernante P, De Palma M, Gulino G, Nasi PG, et al. Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg. 2004;28:271–6. https://doi.org/10.1007/s00268-003-6903-1.
-
Vriens D, Adang EM, Netea-Maier RT, Smit JW, de Wilt JH, Oyen WJ, et al. Cost-effectiveness of FDG-PET/CT for cytologically indeterminate thyroid nodules: a decision analytic approach. J Clin Endocrinol Metab. 2014;99:3263–74. https://doi.org/10.1210/jc.2013-3483.
-
Merten MM, Castro MR, Zhang J, Durski JM, Ryder M. Examining the role of preoperative positron emission tomography/computerized tomography (PET/CT) in combination with ultrasonography in discriminating benign from malignant cytologically indeterminate thyroid nodules. Thyroid. 2017;27:95–102. https://doi.org/10.1089/thy.2016.0379.
-
Piccardo A, Puntoni M, Dezzana M, Bottoni G, Foppiani L, Marugo A, et al. Indeterminate thyroid nodules. The role of (18)F-FDG PET/CT in the “era” of ultrasonography risk stratification systems and new thyroid cytology classifications. Endocrine. 2020;69:553–61. https://doi.org/10.1007/s12020-020-02239-y.
-
Evidence based nation-wide guideline thyroid carcinoma version 2.0. 2014 ed. Rotterdam, the Netherlands: Integraal Kankercentrum Nederland; 2014. https://richtlijnendatabase.nl/richtlijn/schildkliercarcinoom/algemeen.html. Accessed 7 July 2021.
-
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. https://doi.org/10.1007/s00259-014-2961-x.
-
Giovanella L, Avram AM, Iakovou I, Kwak J, Lawson SA, Lulaj E, et al. EANM practice guideline/SNMMI procedure standard for RAIU and thyroid scintigraphy. Eur J Nucl Med Mol Imaging. 2019;46:2514–25. https://doi.org/10.1007/s00259-019-04472-8.
-
EQ-5D-5L User Guide. Rotterdam, the Netherlands: EuroQol Research Foundation; 2019. https://euroqol.org/publications/user-guides/. Accessed 7 July 2021.
-
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727–36. https://doi.org/10.1007/s11136-011-9903-x.
-
Bouwmans C, Krol M, Severens H, Koopmanschap M, Brouwer W, Hakkaart-van RL. The iMTA productivity cost questionnaire: a standardized instrument for measuring and valuing health-related productivity losses. Value in health. 2015;18:753–8. https://doi.org/10.1016/j.jval.2015.05.009.
-
iMTA Productivity and Health Research Group. Manual iMTA medical cost questionnaire (iMCQ). Rotterdam: Institute for Medical Technology Assessment (iMTA), Erasmus university Rotterdam. Rotterdam. https://www.imta.nl/. Accessed 14 April 2021.
-
Dutch Healthcare Authority (NZa). Open data of the Dutch Healthcare Authority (NZa). Utrecht, the Netherlands: Dutch Healthcare Authority (NZa); 2020. https://opendisdata.nl/. Accessed 14 April 2021.
-
Hakkaart-van Roijen L, van der Linden N, Bouwmans CAM, Kanters T, Tan SS. Costing manual: methodology of costing research and reference prices for economic evaluations in healthcare. Rotterdam, the Netherlands: Institute for Medical Technology Assessment (iMTA), Erasmus University Rotterdam; 2015.
-
Haugen BR, Sawka AM, Alexander EK, Bible KC, Caturegli P, Doherty GM, et al. American thyroid association guidelines on the management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2017;27:481–3. https://doi.org/10.1089/thy.2016.0628.
-
Lloyd RV, Osamura RY, Klöppel G, Rosai J. 2017 WHO classification of tumours of endocrine organs. WHO Classification of Tumours, 4th ed. Lyon, France: IARC
-
Kahan BC, Morris TP. Reporting and analysis of trials using stratified randomisation in leading medical journals: review and reanalysis. BMJ. 2012;345: e5840. https://doi.org/10.1136/bmj.e5840.
-
Pathak KA, Klonisch T, Nason RW, Leslie WD. FDG-PET characteristics of Hurthle cell and follicular adenomas. Ann Nucl Med. 2016;30:506–9. https://doi.org/10.1007/s12149-016-1087-6.
-
Ceriani L, Milan L, Virili C, Cascione L, Paone G, Trimboli P, et al. Radiomics analysis of [(18)F]-fluorodeoxyglucose-avid thyroid incidentalomas improves risk stratification and selection for clinical assessment. Thyroid. 2020. https://doi.org/10.1089/thy.2020.0224.
-
Giovanella L, Milan L, Piccardo A, Bottoni G, Cuzzocrea M, Paone G, et al. Radiomics analysis improves (18)FDG PET/CT-based risk stratification of cytologically indeterminate thyroid nodules. Endocrine. 2021. https://doi.org/10.1007/s12020-021-02856-1.
-
de Koster EJ, de Geus-Oei LF, Dekkers OM, van Engen-van GI, Hamming J, Corssmit EPM, et al. Diagnostic utility of molecular and imaging biomarkers in cytological indeterminate thyroid nodules. Endocr Rev. 2018;39:154–91. https://doi.org/10.1210/er.2017-00133.
-
Gopal RK, Kubler K, Calvo SE, Polak P, Livitz D, Rosebrock D, et al. Widespread chromosomal losses and mitochondrial DNA alterations as genetic drivers in Hurthle cell carcinoma. Cancer Cell. 2018;34:242–55. https://doi.org/10.1016/j.ccell.2018.06.013.
-
Doerfler WR, Nikitski AV, Morariu EM, Ohori NP, Chiosea SI, Landau MS, et al. Molecular alterations in Hurthle cell nodules and preoperative cancer risk. Endocr Relat Cancer. 2021. https://doi.org/10.1530/ERC-20-0435.
-
Rosario PW, Rocha TG, Calsolari MR. Fluorine-18-fluorodeoxyglucose positron emission tomography in thyroid nodules with indeterminate cytology: a prospective study. Nucl Med Commun. 2019;40:185–7. https://doi.org/10.1097/MNM.0000000000000946.
-
Qichang W, Jinming S, Lu L, Bin J, Renjie W, Xiuying Z. Comparison of 18F-FDG-PET and 18F-FDG-PET/CT for the diagnostic performance in thyroid nodules with indeterminate cytology: a meta-analysis. Medicine. 2020;99:1–9. https://doi.org/10.1097/MD.0000000000020446.
-
Kaida H, Hiromatsu Y, Kurata S, Kawahara A, Hattori S, Taira T, et al. Relationship between clinicopathological factors and fluorine-18-fluorodeoxyglucose uptake in patients with papillary thyroid cancer. Nucl Med Commun. 2011;32:690–8. https://doi.org/10.1097/MNM.0b013e32834754f1.
-
Staibano P, Forner D, Noel CW, Zhang H, Gupta M, Monteiro E, et al. Ultrasonography and fine-needle aspiration in indeterminate thyroid nodules: a systematic review of diagnostic test accuracy. Laryngoscope. 2021. https://doi.org/10.1002/lary.29778.
-
Yip L, Sosa JA. Molecular-directed treatment of differentiated thyroid cancer: advances in diagnosis and treatment. JAMA Surg. 2016;151:663–70. https://doi.org/10.1001/jamasurg.2016.0825.
-
Steward DL, Carty SE, Sippel RS, Yang SP, Sosa JA, Sipos JA, et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: a prospective blinded multicenter study. JAMA Oncol. 2018;5:204–12. https://doi.org/10.1001/jamaoncol.2018.4616.
-
Angell TE, Heller HT, Cibas ES, Barletta JA, Kim MI, Krane JF, et al. Independent comparison of the Afirma genomic sequencing classifier and gene expression classifier for cytologically indeterminate thyroid nodules. Thyroid. 2019;29:650–6. https://doi.org/10.1089/thy.2018.0726.
-
Eszlinger M, Bohme K, Ullmann M, Gorke F, Siebolts U, Neumann A, et al. Evaluation of a Two-year routine application of molecular testing of thyroid fine-needle aspirations using a seven-gene panel in a primary referral setting in Germany. Thyroid. 2017;27:402–11. https://doi.org/10.1089/thy.2016.0445.
-
Bardet S, Goardon N, Lequesne J, Vaur D, Ciappuccini R, Leconte A, et al. Diagnostic and prognostic value of a 7-panel mutation testing in thyroid nodules with indeterminate cytology: the SWEETMAC study. Endocrine. 2021;71:407–17. https://doi.org/10.1007/s12020-020-02411-4.
-
Paschke R, Cantara S, Crescenzi A, Jarzab B, Musholt TJ, Sobrinho SM. European thyroid association guidelines regarding thyroid nodule molecular fine-needle aspiration cytology diagnostics. Eur Thyroid J. 2017;6:115–29. https://doi.org/10.1159/000468519.
-
Balentine CJ, Vanness DJ, Schneider DF. Cost-effectiveness of lobectomy versus genetic testing (Afirma(R)) for indeterminate thyroid nodules: considering the costs of surveillance. Surgery. 2018;163:88–96. https://doi.org/10.1016/j.surg.2017.10.004.
-
Nicholson KJ, Roberts MS, McCoy KL, Carty SE, Yip L. Molecular testing versus diagnostic lobectomy in Bethesda III/IV thyroid nodules: a cost-effectiveness analysis. Thyroid. 2019;29:1237–43. https://doi.org/10.1089/thy.2018.0779.
-
Endo M, Porter K, Long C, Azaryan I, Phay JE, Ringel MD, et al. Features of cytologically indeterminate molecularly benign nodules treated with surgery. J Clin Endocrinol Metab. 2020;105:e3971–80. https://doi.org/10.1210/clinem/dgaa506.
-
Wang H, Dai H, Li Q, Shen G, Shi L, Tian R. Investigating (18)F-FDG PET/CT parameters as prognostic markers for differentiated thyroid cancer: a systematic review. Front Oncol. 2021;11:1–9. https://doi.org/10.3389/fonc.2021.648658.
-
Choi JW, Yoon YH, Yoon YH, Kim SM, Koo BS. Characteristics of primary papillary thyroid carcinoma with false-negative findings on initial (18)F-FDG PET/CT. Ann Surg Oncol. 2011;18:1306–11. https://doi.org/10.1245/s10434-010-1469-2.
Acknowledgements
The authors like to thank all the patients who participated in the EfFECTS trial, all members of the EfFECTS trial consortium, and all others who were involved in any of the study procedures. In particular, we like to thank Dr. B. Küsters (BK), a pathologist at the Radboud university medical centre, Nijmegen, the Netherlands, for his assistance with the central review of cytology and histopathology samples, and Prof. dr. O.M. Dekkers, a clinical epidemiologist and endocrinologist at the Leiden University Medical Center, Leiden, the Netherlands, and Prof. dr. H. Putter, a statistician at the Leiden University Medical Center, Leiden, the Netherlands, for their expertise and guidance during the execution of the trial.
Funding
The EfFECTS trial was supported by a project grant from the Dutch Cancer Society (KUN 2014–6514).
Author information
Affiliations
Consortia
Contributions
Lioe-Fee de Geus-Oei, Wim J.G. Oyen, and Dennis Vriens conceptualised the study. Lioe-Fee de Geus-Oei was the project leader. Wim J.G. Oyen and Dennis Vriens were principal investigators. Elizabeth J. de Koster was the junior investigator. Adrienne H. Brouwers, Eveline W.C.M. van Dam, Lioe-Ting Dijkhorst-Oei, Tamira K. Klooker, Romana T. Netea-Maier, and Marieke Snel were local principal investigators in hospitals participating in the study. Adriana C.H. van Engen-van Grunsven was the principal central pathologist. Elizabeth J. de Koster prepared the dataset for analysis, drafted the manuscript and prepared the tables and figures. Elizabeth J. de Koster and Dennis Vriens verified the data and performed the statistical analysis. All authors contributed to data acquisition and the interpretation of the data, and critically reviewed this manuscript. All authors had full access to all the data in the study and approved the manuscript before submission. Dennis Vriens had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Ethics approval
The study protocol was approved by the Medical Research Ethics Committee on Research Involving Human Subjects region Arnhem-Nijmegen, Nijmegen, the Netherlands, on 10 November 2014.
Consent to participate
Prior to any study activities, written informed consent was obtained from all individual participants included in the study.
Consent for publication
All patients signed informed consent regarding publishing their data.
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Endocrinology.
Gerelateerde artikelen
- Cryo-ablatie = bevriezing van kwaadaardig tumorweefsel blijkt succesvolle behandeling bij 2 patienten met schildklierkanker uitgevoerd in UMC Maastricht
- Immuuntherapie met Atezolizumab naast een gerichte behandeling met vemurafenib + cobimetinib verbetert sterk de overall overleving bij anaplastische schildklierkanker
- Theranostiek, succesvol bij prostaatkanker en schildklierkanker lijkt ook interessante behandeling voor borstkanker te kunnen zijn, aldus prof. dr. Dalm uit Erasmus MC copy 1
- [18F]FDG-PET/CT-gestuurde diagnose van verdachte schildklierknobbeltjes vermindert nutteloze operaties met 40 procent door goedaardig van kwaadaardig te onderscheiden.
- Schildklierkanker: Bestraling voor andere vormen van kanker veroorzaakt meer en ernstiger vorm van schildklierkanker.
- Selpercatinib (LOXO-292), geeft alsnog uitstekende resultaten (68 procent objectieve respons) met langdurende remissies bij zwaar voorbehandelde RET fusie-positieve niet-kleincellige longkanker met ook hersenuitzaaiingen copy 2
- Oudere patiënten (40 plus) met primaire papillaire schildklierkanker in een actief wait-and-see programma hebben een lager risico op tumorgroei en ziekteprogressie in vergelijking met jongere patienten
- Vier internationale medisch-specialistische verenigingen stellen zorgplan op voor optimale behandelingen voor patiënten met gedifferentieerde schildklierkanker (DTC)
- Anaplastische schildklierkanker (ATC) is een zeldzame vorm van kanker en heel moeilijk te behandelen maar immuuntherapie en gerichte behandelingen op mutaties en eiwitexpressie geven hoopvolle resultaten
- Schildklierkanker: Lenvatinib geeft zeer goede resultaten bij uitbehandelde gevorderde, uitgezaaide schildklierkanker waarbij mediane overleving stijgt van 3,5 naar 18,5 maanden binnen studie van 2 jaar. copy 1
- Wie als baby/kind bestraald is voor een vergrote schildklier loopt sterk vergroot risico op schildklierkanker. Dit risico blijft het hele leven bestaan blijkt uit studie na 50 jaar copy 1



Plaats een reactie ...
Reageer op "[18F]FDG-PET/CT-gestuurde diagnose van verdachte schildklierknobbeltjes vermindert nutteloze operaties met 40 procent door goedaardig van kwaadaardig te onderscheiden."