10 december 2018: lees ook dit artikel:
29 maart 2018: Zie ook dit artikel:
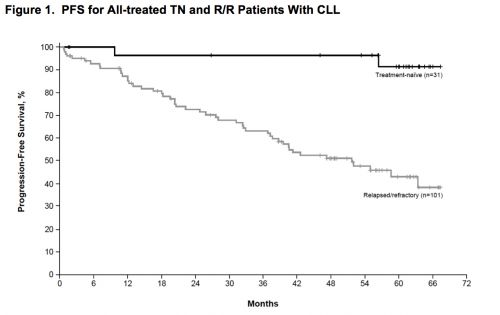
4 mei 2017: Ibrutinib geeft op 5-jaars meting 89 procent PR = gedeeltelijke remissies waarvan 29 procent CR = complete remissies.
Lange termijn resultaten van ibrutinib (Imbruvica) bij patiënten met eerder onbehandelde of met chemo behandelde Chronische lymfatische leukemie (CLL) of (SLL) toont veel duurzame remissies. Op 5 jaars meting vertoonde 89 procent van de patiënten een remissie van minimaal 50 procent. 29 procent had een CR = complete remissie bereikt en waren op 5-jaars meting dus klinisch kankervrij. En belangrijk zonder ernstige bijwerkingen op langere termijn.
“This is the longest follow-up in any CLL population. Responses are impressive with single-agent ibrutinib upfront, and the drug is generally well tolerated,” zegt hoofdonderzoeker Susan O’Brien, MD, Associate Director for Clinical Science, Chao Family Comprehensive Cancer Center, University of California Irvine Health. “Importantly, there were no late unexpected side effects.”
KERNPUNTEN:
Clinical Trials of Ibrutinib and Venetoclax in CLL/SLL
- At 5 years, 89% of patients with treatment-naive and relapsed or refractory CLL/SLL achieved a complete response or partial response to ibrutinib.
- The least favorable outcome with ibrutinib was seen in patients with 17p deletion.
- Venetoclax may be an option for high-risk patients who relapse or are refractory to ibrutinib.
- There may still be a role for chemotherapy as upfront treatment of younger fit patients with mutated IGHV.
Interessant si om ook deze studie: Spotlight on ibrutinib and its potential in frontline treatment of chronic lymphocytic leukemia eens te bekijken:
Table 2
Studies with ibrutinib as frontline treatment for chronic lymphocytic leukemia
| Study | No of patients | R/R | Treatment | Median age, range (years) | ORR% | PFS (months) | OS (months) |
|---|---|---|---|---|---|---|---|
| Burger et al29 | 40 | Yes: 36 No: 4a |
Ibrutinib + rituximab | 63 (35–82) | 95 | 18 (78%) | 18 (83.8%) |
| O’Brien et al36 | 31 | No | Ibrutinib (low dose vs high dose) | 71 (65–84) | 71 | 24 (96%) | 24 (96%) |
| Byrd et al39 | 31b | No | Ibrutinib | 71 (65–84) | 84 | 20 (96%) | 20 (97%) |
| Farooqui38 | 33b | No | Ibrutinib (untreated vs treated) | 62 (33–82) vs 62 (56–79) | 97 | 24 (91% vs 80%) | 24 (84% vs 74%) |
| Burger et al31 | 269 | No | Ibrutinib vs chlorambucil | 73 (65–89) vs 72 (65–90) | 86 vs 35 | 18 (90% vs 52%), P<0.001 | 24 (98% vs 85%), P=0.001 |
Notes:
bData only of those patients receiving initial therapy with ibrutinib are shown
Bovenstaande is een sterk verkorte weergave van de resultaten, ook uit deze drie studies:
References
Zie verder dit artikel van vorig jaar:
15 maart 2016: Bron: The Lancet, 2016 Feb;17(2):200-11. doi: 10.1016/S1470-2045(15)00465-9. Epub 2015 Dec 5.
Hoewel de combinatiebehandeling bendamustine en rituximab al voor goede resultaten zorgt bij met name Chronische Lympfatische Leukemie en lymfklierkanker met kleine meetbare tumoren (>1,5 cm.) in vergelijking met bv. de CHOP kuren blijkt Ibrutinib als extra medicijn de progressievrije ziekte en overall overleving nog eens met tientallen procenten te verbeteren.
Na 1,5 jaar was het verschil in progressievrije overleving tussen Ibrutinib plus bendamustine en rituximab versus placebo plus bendamustine en rituximabal 79% vs 24%. (HR = 0.203, P < .0001)
De mediane progressievrije ziekte was in de ibrutinib groep nog niet bereikt versus 13.3 maanden in placebogroep (95% confidence interval = 11.3–13.9 maanden)(hazard ratio = 0.203, P < .0001).
Dit blijkt uit een grote gerandomiseerde placebo gecontroleerde Fase III studie bij totaal 578 patiënten met CLL - Chronische Lymfatische Leukemie of recidief van meetbare lymfklierkanker ((>1,5 cm) verdeeld dus over twee groepen: ibrutinib N = 289) of placebo (N = 289) en gepubliceerd in The Lancet.
Bovenstaande grafiek is uit: Independent evaluation of ibrutinib efficacy 3 years post-initiation of monotherapy in patients with chronic lymphocytic leukemia/small lymphocytic leukemia including deletion 17p disease.
Conclusie: The addition of ibrutinib to bendamustine/rituximab increased progression-free survival in patients with chronic lymphocytic leukemia or small lymphocytic lymphoma relapsing after initial therapy. Improvements were consistent across high-risk subgroups.
Het volledige studierapport: Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. is tegen betaling in te zien.
Hier het originele abstract van de studie:
In patients eligible for bendamustine plus rituximab, the addition of ibrutinib to this regimen results in significant improvements in outcome with no new safety signals identified from the combination and a manageable safety profile.
Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study.
Abstract
BACKGROUND:
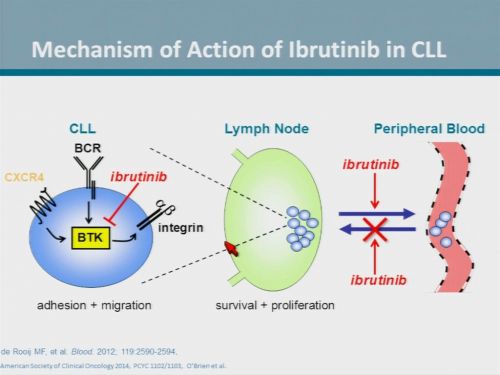
Most patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma relapse after initial therapy. Bendamustine plus rituximab is often used in the relapsed or refractory setting. We assessed the efficacy and safety of adding ibrutinib, an oral covalent inhibitor of Bruton's tyrosine kinase (BTK), to bendamustine plus rituximab in patients with previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma.
METHODS:
The HELIOS trial was an international, double-blind, placebo-controlled, phase 3 study in adult patients (≥18 years of age) who had active chronic lymphocytic leukaemia or small lymphocytic lymphoma with measurable lymph node disease (>1·5 cm) by CT scan, and had relapsed or refractory disease following one or more previous lines of systemic therapy consisting of at least two cycles of a chemotherapy-containing regimen, an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1, and adequate bone marrow, liver, and kidney function. Patients with del(17p) were excluded because of known poor response to bendamustine plus rituximab. Patients who had received previous treatment with ibrutinib or other BTK inhibitors, refractory disease or relapse within 24 months with a previous bendamustine-containing regimen, or haemopoietic stem-cell transplant were also excluded. Patients were randomly assigned (1:1) by a web-based system to receive bendamustine plus rituximab given in cycles of 4 weeks' duration (bendamustine: 70 mg/m(2) intravenously on days 2-3 in cycle 1, and days 1-2 in cycles 2-6; rituximab: 375 mg/m(2) on day 1 of cycle 1, and 500 mg/m(2) on day 1 of cycles 2-6 for a maximum of six cycles) with either ibrutinib (420 mg daily orally) or placebo until disease progression or unacceptable toxicity. Patients were stratified according to whether they were refractory to purine analogues and by number of previous lines of therapy. The primary endpoint was independent review committee (IRC)-assessed progression-free survival. Crossover to ibrutinib was permitted for patients in the placebo group with IRC-confirmed disease progression. Analysis was by intention-to-treat and is continuing for further long-term follow-up. The trial is registered with ClinicalTrials.gov, number NCT01611090.
FINDINGS:
Between Sept 19, 2012, and Jan 21, 2014, 578 eligible patients were randomly assigned to ibrutinib or placebo in combination with bendamustine plus rituximab (289 in each group). The primary endpoint was met at the preplanned interim analysis (March 10, 2015). At a median follow-up of 17 months (IQR 13·7-20·7), progression-free survival was significantly improved in the ibrutinib group compared with the placebo group (not reached in the ibrutinib group (95% CI not evaluable) vs 13·3 months (11·3-13·9) in the placebo group (hazard ratio 0·203, 95% CI 0·150-0·276; p<0·0001). IRC-assessed progression-free survival at 18 months was 79% (95% CI 73-83) in the ibrutinib group and 24% (18-31) in the placebo group (HR 0·203, 95% CI 0·150-0·276; p<0·0001). The most frequent all-grade adverse events were neutropenia and nausea. 222 (77%) of 287 patients in the ibrutinib group and 212 (74%) of 287 patients in the placebo group reported grade 3-4 events; the most common grade 3-4 adverse events in both groups were neutropenia (154 [54%] in the ibrutinib group vs 145 [51%] in the placebo group) and thrombocytopenia (43 [15%] in each group). A safety profile similar to that previously reported with ibrutinib and bendamustine plus rituximab individually was noted.
INTERPRETATION:
In patients eligible for bendamustine plus rituximab, the addition of ibrutinib to this regimen results in significant improvements in outcome with no new safety signals identified from the combination and a manageable safety profile.
FUNDING:
Janssen Research & Development.
Copyright © 2016 Elsevier Ltd. All rights reserved.
- PMID:
- 26655421
- [PubMed - in process]
Gerelateerde artikelen
- Pirtobrutinib, een niet-covalente BTK remmer, blijkt effectief bij zwaar voorbehandelde patiënten met chronische lymfatische leukemie (CLL) of kleincellig lymfatisch lymfoom (SLL) die eerder een covalente BTK remmer hadden gehad
- Reviewstudie beschrijft hoe je het beste patienten met CLL = Chronische Lymfatische Leukemie kunt diagnosteren en welke behandelingen het beste zijn te gebruiken in verschillende stadia van de ziekte.
- Venetoclax plus rituximab geeft ook bij jonge en fitte patiënten met chronische lymfatische leukemie en zelfs bij ongemuteerde IGHV veel complete remissies
- acalabrutinib als monotherapie verbeterde progressievrije overleving aanzienlijk met lager bijwerkingenprofiel dan idelalisib + rituximab of bendamustine + rituximab bij gevorderde CLL - chronische lymfatische leukemie
- Ibrutinib geeft veel betere resultaten op ziekteprogressievrije overleving (87 vs 74 procent) dan bendamustine plus rituximab bij onbehandelde oudere CLL patienten. Rituximab lijkt zinloos naast ibrutinib
- Man - 48 jaar - met al 20 jaar Chronische Lymfatische Leukemie (CLL) weigert chemo en bereikt een langdurige en duurzame remissie na behandeling met Epigallocatechin-3-gallate, een extract van groene thee. copy 1
- BCL-2 remmer - ABT-199/GDC-0199 geeft spectaculaire resultaten bij vergevorderde zwaar voorbehandelde CLL -Chronische Lymfatische Leukemie met alsnog 23 procent totale remissies van minimaal 2 jaar
- Ibrutinib geeft veel betere ziektevrije tijd (plus 45 procent) in vergelijking met rituximab bij recidief van chronische lymfatische leukemie en SLL - lymfklierkanker
- Ibrutinib aanvullend op Bendamustine en Rituximab verbetert progressievrije ziekte en overall overleving met tientallen procenten bij Chronische Lymfatische Leukemia en recidief van lymfklierkanker met kleine tumoren copy 1
- Ibrutinib - Tyrosine kinase (BTK) remmer PCI 32.765 - Ibrutinib zorgt voor langere ziektevrije tijd met veel minder bijwerkingen bij patiënten met CLL - chronische lymfatische leukemie bevestigen nieuwe studies copy 1
- Idelalisib naast rituximab zorgt voor significant langere overleving en progressievrije tijd bij patienten met CLL - chronische lymfatische leukemie waarvoor chemo niet meer mogelijk was
- Groene thee extract zorgt binnen enkele maanden voor significante remissie bij CLL patienten (Chronische Lymfatische Leukemie) . Nieuwe fase II studie bevestigt eerdere goede resultaten. copy 1
- Ofatumumab door FDA goedgekeurd als medicijn voor patienten met CLL - Chronische Lymfatische Leukemie, ongevoelig geworden voor fludarabine en alemtuzumab.
- Combinatiebehandeling van fludarabine en alemtuzumab geeft significant langere ziektevrije tijd en betere overleving voor eerder behandelde CLL patienten
- Rituximab naast Fludarabine en cyclophosphamide geeft significant betere resultaten op ziektevrije tijd en op overleving bij CLL en wordt eerste lijns.
- CLL - Chronische Lymfatische Leukemie: een overzicht



Plaats een reactie ...
Reageer op "Ibrutinib aanvullend op Bendamustine en Rituximab verbetert progressievrije ziekte en overall overleving met tientallen procenten bij Chronische Lymfatische Leukemia en recidief van lymfklierkanker met kleine tumoren copy 1"