Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ook via uw lidmaatschapsnummer korting krijgen bij enkele bedrijven.
En raadpleeg ook literatuurlijst niet-toxische middelen en behandelingen specifiek bij borstkanker van arts-bioloog drs. Engelbert Valstar
En zie ook onder preventie bij borstkanker bij fysieke activiteiten
19 september 2018: Zie ook deze studie: Physical Activity and Survival After Breast Cancer Diagnosis die de meerwaarde van fysieke activiteit bij borstkanker aantoont. Zoals meerdere studies dit aantonen, zie ook onze literatuurlijst specifiek bij borstkanker van arts-bioloog drs. Engelbert Valstar
17 januari 2017: Bron: Cancer Causes Control. 2011 Mar; 22(3): 427–435.
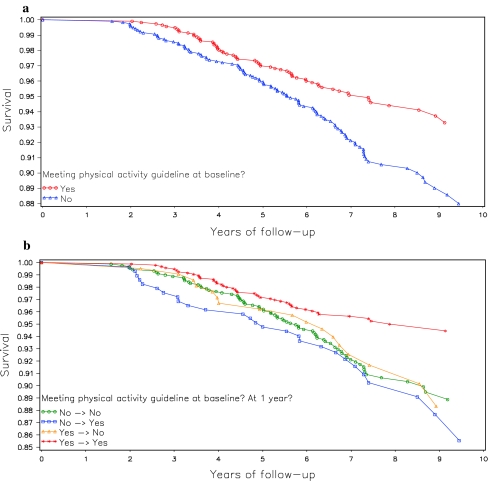
Fysieke activiteit zoals bewust bewegen en sporten heeft grote invloed op het risico aan overlijden voor borstkankerpatiënten die 1 jaar na hun behandelingen nog in leven zijn. Borstkankerpatienten die bij de start van de studie al veel actief waren en dit door bleven zetten tijdens en na hun behandelingen hadden 52% minder risico op overlijden in vergelijking met borstkankerpatiënten die weinig fysiek actief waren voor en tijdens hun behandelingen en daar ook niets aan deden. Vrouwen die bij de start weinig actief waren maar na hun behandelingen wel fysiek actiever werden reduceerden daarmee hun kans op overlijden met 35% tot 42%. Opvallend was dat de vrouwen die niets veranderden in hun fysieke activiteiten na 1 jaar ook geen wezenlijke veranderingen zagen in hun kans op overlijden. Dus borstkankerpatiënten die toch al wel fysiek actief waren bleven zelfde minder risico op overlijden van ca. 50% te hebben in vergelijking met borstkankerpatienten die weinig fysiek actief waren zowel bij de start als daarna daar niets aan veranderden.
Dit betekent dat op elk moment na behandeling van borstkanker het goed is om fysiek actiever te worden en bv. mee te gaan doen aan bewegingsprogramma's die overal worden aangeboden. Of gewoon zelf meer gaan bewegen, wandelen, sporten enz. Het heeft echt gote invloed op de overall overleving.
Hier de grafiek in overall overleving: (tekst gaat onder grafiek en tabellen verder)
Hier achtereenvolgens wat tabellen uit de studie:
Table 1
Baseline characteristics of WHEL participants (N = 2,361) stratified by adherence to physical activity guidelines
| Meeting guidelines | Not meeting guidelines | p for difference |
|---|
| N | Mean (SD) or % | N | Mean (SD) or % |
|---|
| Age, mean (SD) |
1,175 |
54.3 (9.1) |
1,186 |
53.5 (8.6) |
.04 |
| Age group (%) |
| <44 |
154 |
13.1% |
155 |
13.1% |
.02 |
| 45–54 |
434 |
36.9% |
499 |
42.1% |
|
| 55–65 |
411 |
35.0% |
395 |
33.3% |
|
| >65 |
176 |
15.0% |
137 |
11.6% |
|
| Race/ethnicity |
| Non-Hispanic White |
1,055 |
89.8% |
988 |
83.3% |
<.0001 |
| African-American |
23 |
2.0% |
47 |
4.0% |
|
| Hispanic-American |
40 |
3.4% |
70 |
5.9% |
|
| Asian-American |
37 |
3.2% |
45 |
3.8% |
|
| Other |
20 |
1.7% |
36 |
3.0% |
|
| College graduate (%) |
746 |
63.5% |
562 |
47.4% |
<.0001 |
| BMI, mean kg/m2 (SD) |
1,175 |
25.6 (4.7) |
1,186 |
28.5 (6.6) |
<.0001 |
| BMI category (%) |
| <25.0 |
617 |
52.5% |
422 |
35.6% |
<.0001 |
| 25–29.9 |
375 |
31.9% |
361 |
30.4% |
|
| ≥30.0 |
183 |
15.6% |
403 |
34.0% |
|
| Menopausal status |
| Post-menopausal |
934 |
79.5% |
970 |
81.8% |
<.0001 |
| Pre-menopausal |
125 |
10.6% |
107 |
9.0% |
|
| Peri-menopausal |
114 |
9.7% |
109 |
9.2% |
|
| Hot flashes (%) |
349 |
29.7% |
357 |
30.1% |
.98 |
| Time since diagnosis |
| <2 years |
638 |
54.3% |
652 |
55.0% |
.74 |
| 2–4 years |
537 |
45.7% |
534 |
45.0% |
|
| Tumor stage (%) |
| I |
514 |
43.7% |
443 |
37.4% |
.003 |
| IIA |
378 |
32.2% |
396 |
33.4% |
|
| IIB |
121 |
10.3% |
172 |
14.5% |
|
| IIIA |
131 |
11.2% |
135 |
11.4% |
|
| IIIC |
31 |
2.6% |
40 |
3.4% |
|
| Chemotherapy (%) |
759 |
64.6% |
852 |
71.8% |
.0008 |
| Radiation (%) |
720 |
61.3% |
734 |
61.9% |
.95 |
| # positive nodes (%) |
| 0 |
730 |
62.1% |
671 |
56.6% |
.03 |
| 1–3 |
304 |
25.9% |
365 |
30.8% |
|
| >3 |
141 |
12.9% |
149 |
12.6% |
|
| Anti-estrogen use (%) |
836 |
71.5% |
759 |
65.0% |
.0007 |
| Smoking status |
636 |
54.1% |
663 |
55.9% |
.01 |
| Never |
508 |
43.2% |
468 |
39.5% |
|
| Former |
31 |
2.6% |
55 |
4.6% |
|
| Current |
|
|
|
|
|
| Alcohol (%) |
| 0 |
339 |
28.9% |
380 |
32.1% |
.21 |
| 1–19 g/day |
786 |
67.0% |
761 |
64.3% |
|
| 20+ g/day |
48 |
4.1% |
42 |
3.6% |
|
En hier de tabel met de verschillen in kans op overlijden en recidief verdeeld in vier kwarten:
Table 2
Adjusted analyses for physical activity, change in activity level additional breast cancer events, and death from all causes in a cohort of women with a history of breast cancer (n = 2,361)
| Physical activity (MET-h/week) | N | n | Additional breast cancer events | n | All-cause mortality | p (trend) |
|---|
| HR (95% CI) | p | p (trend) | HR (95% CI) | p |
|---|
| Baseline activity level |
| Total PA (continuous) |
2,361 |
295 |
0.99 (0.99, 1.00) |
.21 |
|
163 |
0.98 (0.96, 0.99) |
.003 |
|
| Total PA (categorical) |
| Quintile 1 [0–2.5] |
481 |
69 |
Reference |
|
.58 |
43 |
Reference |
|
.08 |
| Quintile 2 (2.5–7.5] |
485 |
65 |
0.91 (0.64, 1.28) |
.58 |
|
43 |
1.01 (0.66, 1.55) |
.97 |
|
| Quintile 3 (7.5–14.9] |
451 |
53 |
0.85 (0.59, 1.22) |
.38 |
|
32 |
0.85 (0.53, 1.35) |
.48 |
|
| Quintile 4 (14.9–24.7] |
472 |
60 |
0.97 (0.68, 1.39) |
.87 |
|
27 |
0.75 (0.46, 1.23) |
.26 |
|
| Quintile 5 (24.7–107] |
472 |
48 |
0.74 (0.50, 1.10) |
.13 |
|
18 |
0.47 (0.26, 0.84) |
.01 |
|
| Mod-vig PA (continuous) |
2,361 |
295 |
0.99 (0.98, 1.00) |
.13 |
|
163 |
0.98 (0.96, 0.99) |
.002 |
|
| Mod-vig PA (categorical) |
| Quintile 1 [0–1.3] |
474 |
70 |
Reference |
|
.30 |
45 |
Reference |
|
.02 |
| Quintile 2 (1.3–6.3] |
473 |
64 |
0.97 (0.68, 1.36) |
.84 |
|
38 |
1.00 (0.64, 1.55) |
.99 |
|
| Quintile 3 (6.3–12.5] |
474 |
64 |
0.98 (0.69, 1.38) |
.89 |
|
41 |
0.99 (0.64, 1.54) |
.96 |
|
| Quintile 4 (12.5–22.9] |
470 |
53 |
0.91 (0.62, 1.31) |
.60 |
|
24 |
0.71 (0.42, 1.19) |
.19 |
|
| Quintile 5 (22.9–107] |
470 |
44 |
0.67 (0.45, 1.00) |
.04 |
|
15 |
0.39 (0.21, 0.72) |
.003 |
|
| Meeting PA guidelinea |
| No |
1,186 |
161 |
Reference |
|
|
102 |
Reference |
|
|
| Yes |
1,175 |
134 |
0.89 (0.70, 1.14) |
.36 |
|
61 |
0.65 (0.47, 0.91) |
.01 |
|
| Baseline-to-1-year change |
| Meeting PA guideline |
| No → No |
899 |
112 |
Reference |
|
|
75 |
Reference |
|
|
| No → Yes |
287 |
49 |
1.44 (1.02, 2.03) |
.04 |
|
27 |
1.21 (0.77, 1.90) |
.42 |
|
| Yes → No |
213 |
31 |
1.22 (0.81, 1.83) |
.34 |
|
17 |
1.04 (0.61, 1.77) |
.89 |
|
| Yes → Yes |
962 |
103 |
0.93 (0.70, 1.24) |
.62 |
|
44 |
0.60 (0.40, 0.88) |
.01 |
|
| Δ Total PA (continuous) |
2,361 |
295 |
1.01 (1.00, 1.02) |
.26 |
|
163 |
1.00 (0.98, 1.01) |
.59 |
|
| Δ Total PA (categorical) |
| Quintile 1 [−68.8, −5.5] |
474 |
52 |
Reference |
|
.60 |
23 |
Reference |
|
.70 |
| Quintile 2 (−5.5, −0.3] |
473 |
58 |
0.95 (0.64, 1.42) |
.81 |
|
39 |
1.24 (0.71, 2.16) |
.45 |
|
| Quintile 3 (−0.3, 2.3] |
474 |
65 |
1.24 (0.82, 1.87) |
.31 |
|
40 |
1.23 (0.69, 2.20) |
.49 |
|
| Quintile 4 (2.3, 7.5] |
470 |
57 |
1.10 (0.73, 1.65) |
.66 |
|
35 |
1.17 (0.66, 2.09) |
.60 |
|
| Quintile 5 (7.5, 92.3] |
470 |
63 |
1.19 (0.80, 1.77) |
.39 |
|
26 |
0.89 (0.49, 1.64) |
.71 |
|
| Δ Mod-vig PA (continuous) |
2,361 |
295 |
1.01 (1.00, 1.02) |
.23 |
|
163 |
0.99 (0.98, 1.01) |
.48 |
|
| Δ Mod-vig PA (categorical)b |
| Quintile 1 [−63.8, −5.3] |
476 |
54 |
Reference |
|
.58 |
24 |
Reference |
|
.84 |
| Quintile 2 (−5.3, 0] |
805 |
99 |
0.95 (0.65, 1.40) |
.76 |
|
69 |
1.17 (1.69, 1.97) |
.56 |
|
| Quintile 3 (0, 1.3] |
147 |
18 |
1.00 (0.57, 1.75) |
.99 |
|
9 |
0.90 (0.40, 2.02) |
.81 |
|
| Quintile 4 (1.3, 7) |
563 |
55 |
0.99 (0.66, 1.49) |
.97 |
|
32 |
1.06 (0.60, 1.89) |
.84 |
|
| Quintile 5 [7, 92.3] |
470 |
69 |
1.23 (0.83, 1.80) |
.30 |
|
29 |
0.92 (0.51, 1.66) |
.78 |
|
Higher baseline (post-treatment) physical activity was associated with improved survival. However, change in activity over the following year was not associated with outcomes. These data suggest that long-term physical activity levels are important for breast cancer prognosis.
Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: findings from the WHEL Study
Lisa A. Cadmus Bertram,
1 Marcia L. Stefanick,
2 Nazmus Saquib,
1 Loki Natarajan,
1 Ruth E. Patterson,
1 Wayne Bardwell,
1 Shirley W. Flatt,
1 Vicky A. Newman,
1 Cheryl L. Rock,
1 Cynthia A. Thomson,
3 and
John P. Pierce 1
1
Abstract
Objective
Research suggests that physical activity is associated with improved breast cancer survival, yet no studies have examined the association between post-diagnosis changes in physical activity and breast cancer outcomes. The aim of this study was to determine whether baseline activity and 1-year change in activity are associated with breast cancer events or mortality.
Methods
A total of 2,361 post-treatment breast cancer survivors (Stage I–III) enrolled in a randomized controlled trial of dietary change completed physical activity measures at baseline and one year. Physical activity variables (total, moderate–vigorous, and adherence to guidelines) were calculated for each time point. Median follow-up was 7.1 years. Outcomes were invasive breast cancer events and all-cause mortality.
Results
Those who were most active at baseline had a 53% lower mortality risk compared to the least active women (HR = 0.47; 95% CI: 0.26, 0.84; p = .01). Adherence to activity guidelines was associated with a 35% lower mortality risk (HR = 0.65, 95% CI: 0.47, 0.91; p < .01). Neither baseline nor 1-year change in activity was associated with additional breast cancer events.
Conclusions
Higher baseline (post-treatment) physical activity was associated with improved survival. However, change in activity over the following year was not associated with outcomes. These data suggest that long-term physical activity levels are important for breast cancer prognosis.
Discussion
We found a robust association between level of physical activity at baseline and breast cancer mortality. We did not observe a statistically significant association between baseline physical activity and additional breast cancer events, suggesting that the results for mortality may have been driven by prognosis after an additional event (including a possible effect on type of recurrence) rather than a direct reduction in recurrences or new primaries. Although our analyses used all-cause rather than breast cancer–specific mortality, more than 80% of deaths in the WHEL Study were due to breast cancer [16]; therefore, the observed association between higher baseline physical activity and lower mortality was largely driven by a reduction in breast cancer–related deaths.
Contrary to expectations, changes in physical activity from baseline to 1 year were not associated with survival. It is possible that our 1-year measure of behavior change did not provide a sufficiently long period for the metabolic and physiologic changes associated with increased or decreased physical activity to impact cancer outcomes. Likewise, we observed that the majority of women who adopted physical activity between enrollment and year 1 did not maintain their new activity level over the long term.
Our findings with regard to mortality are generally consistent with previous reports [7, 8]. Similar to the Nurses’ Health Study, the Collaborative Women’s Longevity Study, and the Life after Cancer Epidemiology cohorts, we found that a substantial amount of physical activity was required to improve breast cancer outcomes. An exception is the HEAL Study, which observed a benefit for even very modest levels of physical activity. There are a number of potential explanations for between-study differences in the level of physical activity that is associated with reduced mortality. The cohorts vary somewhat with regard to clinical and other participant characteristics. Perhaps more importantly, the specific approach to physical activity measurement and quantification also varies. For example, the Canadian study assessed a wide variety of physical activity settings (recreational, household, occupational) and categorized results by setting type, whereas some other studies focused only on recreational or leisure-time activity and categorized results by intensity (mild, moderate, vigorous, total). Due to these variations, it is difficult to directly compare studies with regard to the dose of physical activity that is necessary to observe a protective effect on outcomes.
A strength of the WHEL Study was that we assessed physical activity at two time points, allowing us to assess the potential effect of changes in physical activity on breast cancer outcomes. Other strengths included excellent cohort maintenance (health status confirmed at study end confirmed for >95% of participants) and a multi-site, geographically diverse sample of breast cancer survivors.
A limitation of the WHEL Study was the use of self-report of physical activity, which is known to include random error. However, the study used a standardized physical activity questionnaire that was validated against both a physical activity recall procedure and an objective physical activity measure (7-day accelerometer) within a subset of WHEL Study participants [22]. The physical activity questionnaire had good agreement (73%) with the accelerometer measure and had 100% sensitivity for meeting the physical activity guideline. Another limitation is that the WHEL cohort was a fairly racially and ethnically homogenous sample. Thus, findings may not apply to women who are not non-Hispanic White.
Consistent with previous reports, our findings suggest that adherence to physical activity guidelines may improve overall survival among breast cancer survivors. We also present the first examination of post-diagnosis change in physical activity. We observed a 42% reduction in risk mortality among those women who were adhering to physical activity guidelines at both baseline and 1-year relative to those who did not meet the guideline at either time point. However, we did not find that 1-year change in physical activity was associated with breast cancer mortality. These findings highlight the importance of sustained post-diagnosis physical activity and suggest that a 1-year period may be too short for even relatively large increases in physical activity to affect breast cancer outcomes. Long-term exposure appears to be the most important determinant of the relationship between physical activity and breast cancer mortality. To improve breast cancer outcomes, women should be encouraged to maintain an active lifestyle over time, aiming to meet or exceed current physical activity guidelines. Toward this goal, physical activity promotion should be incorporated into breast cancer prevention and control programs. Future studies are needed to further examine the potential effect of long-term post-diagnosis change in physical activity on breast cancer outcomes.
Acknowledgments
The Women’s Healthy Eating and Living (WHEL) Study was initiated with the support of the Walton Family Foundation and continued with funding from National Cancer Institute Grant CA 69375. Some of the data were collected from General Clinical Research Centers, National Institute of Health grants M01-RR00070, M01-RR00079, and M01-RR00827.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
1.
Thune I, Furberg AS. Physical activity and cancer risk: dose-response and cancer, all sites and site-specific. Med Sci Sports Exerc. 2001;33(6 Suppl):S530–S550. [PubMed]2. Sternfeld B, Lee I-M. Physical activity and cancer: the evidence, the issues, and the challenges. In: Lee I-M, editor. Epidemiologic methods in physical activity studies. New York: Oxford University Press; 2008.
3. Bull FC, Armstrong TP, Dixon T, Ham S, Neiman A, Pratt M. Physical inactivity. In: Ezzati M, Lopez AM, Rodgers A, Murray C, editors. Comparative quantification of health risks: global and regional burden of disease attribution to selected major risk factors. Geneva: World Health Organization; 2004.
4.
Monninkhof EM, Elias SG, Vlems FA, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18(1):137–157. doi: 10.1097/01.ede.0000251167.75581.98. [PubMed] [Cross Ref]5.
Emaus A, Veierod MB, Tretli S, et al. Metabolic profile, physical activity, and mortality in breast cancer patients. Breast Cancer Res Treat. 2010;121(3):651–660. doi: 10.1007/s10549-009-0603-y. [PubMed] [Cross Ref]6.
John EM, Horn-Ross PL, Koo J. Lifetime physical activity and breast cancer risk in a multiethnic population: the San Francisco Bay area breast cancer study. Cancer Epidemiol Biomarkers Prev. 2003;12(11 Pt 1):1143–1152. [PubMed]7.
Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Jama. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [PubMed] [Cross Ref]8.
Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379–386. doi: 10.1158/1055-9965.EPI-07-0771. [PubMed] [Cross Ref]9.
Irwin ML, Smith AW, McTiernan A, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol. 2008;26(24):3958–3964. doi: 10.1200/JCO.2007.15.9822. [PMC free article] [PubMed] [Cross Ref]10.
Sternfeld B, Weltzien E, Quesenberry CP, Jr, et al. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):87–95. doi: 10.1158/1055-9965.EPI-08-0595. [PMC free article] [PubMed] [Cross Ref]11.
Friedenreich CM, Gregory J, Kopciuk KA, Mackey JR, Courneya KS. Prospective cohort study of lifetime physical activity and breast cancer survival. Int J Cancer. 2009;124(8):1954–1962. doi: 10.1002/ijc.24155. [PubMed] [Cross Ref]12.
Pasanisi P, Berrino F, Petris M, Venturelli E, Mastroianni A, Panico S. Metabolic syndrome as a prognostic factor for breast cancer recurrences. Int J Cancer. 2006;119(1):236–238. doi: 10.1002/ijc.21812. [PubMed] [Cross Ref]13.
Irwin ML, Varma K, Alvarez-Reeves M, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):306–313. doi: 10.1158/1055-9965.EPI-08-0531. [PMC free article] [PubMed] [Cross Ref]14.
McTiernan A, Ulrich C, Slate S, Potter J. Physical activity and cancer etiology: associations and mechanisms. Cancer Causes Control. 1998;9(5):487–509. doi: 10.1023/A:1008853601471. [PubMed] [Cross Ref]15.
Pierce JP, Faerber S, Wright FA, et al. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women’s Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002;23(6):728–756. doi: 10.1016/S0197-2456(02)00241-6. [PubMed] [Cross Ref]16.
Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. Jama. 2007;298(3):289–298. doi: 10.1001/jama.298.3.289. [PMC free article] [PubMed] [Cross Ref]17.
Gold EB, Flatt SW, Pierce JP, et al. Dietary factors and vasomotor symptoms in breast cancer survivors: the WHEL Study. Menopause. 2006;13(3):423–433. doi: 10.1097/01.gme.0000185754.85328.44. [PubMed] [Cross Ref]18.
Pierce JP, Stefanick ML, Flatt SW, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25(17):2345–2351. doi: 10.1200/JCO.2006.08.6819. [PMC free article] [PubMed] [Cross Ref]19.
McTiernan A, Kooperberg C, White E, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative Cohort Study. Jama. 2003;290(10):1331–1336. doi: 10.1001/jama.290.10.1331. [PubMed] [Cross Ref]20.
Hong S, Bardwell WA, Natarajan L, et al. Correlates of physical activity level in breast cancer survivors participating in the Women’s Healthy Eating and Living (WHEL) Study. Breast Cancer Res Treat. 2007;101(2):225–232. doi: 10.1007/s10549-006-9284-y. [PubMed] [Cross Ref]21.
Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. [PubMed]22.
Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31(2):193–202. [PubMed]23.
Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [PubMed] [Cross Ref]24.
Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2. [PubMed] [Cross Ref]
Our study suggested that exercise intervention was beneficial to breast cancer survivors. Therefore, exercise should be recommended to this patient group.
Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trails
Guoqing Zhu,1 Xiao Zhang,1 Yulan Wang,1 Huizi Xiong,2 Yinghui Zhao,1 and Fenyong Sun1
Abstract
Background
Exercise is associated with favorable outcomes in cancer survivors. The purpose of this meta-analysis is to comprehensively summarize the effects of exercise intervention in breast cancer survivors.
Methods
A systematic search of PubMed, Elsevier, and Google scholar was conducted up to March 2015. References from relevant meta-analyses and reviews were also checked.
Results
Thirty-three randomized controlled trials were included in this meta-analysis, including 2,659 breast cancer survivors. Compared with the control group, quality of life was significantly improved in exercise intervention group, especially in mental health and general health subscales of short form 36 questionnaire, as well as emotion well-being and social well-being subscales of the Functional Assessment of Cancer Therapy. Besides, exercise alleviated the symptoms of depression and anxiety in the exercise group. Furthermore, exercise was also associated with positive outcomes in body mass index, lean mass, and muscle strength. In addition, the serum concentration of insulin, insulin-like growth factor-II, and insulin-like growth factor binding protein-1 was significantly reduced in exercise intervention group. However, based on the current data of this meta-analysis, there were no significant differences in sleep dysfunction or fatigue between groups.
Conclusion
Our study suggested that exercise intervention was beneficial to breast cancer survivors. Therefore, exercise should be recommended to this patient group.
References
1.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. [PubMed]2.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [PubMed]3.
de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22(4):561–570. [PMC free article] [PubMed]4.
Short CE, James EL, Girgis A, Mcelduff P, Plotnikoff RC. Move more for life: the protocol for a randomised efficacy trial of a tailored-print physical activity intervention for post-treatment breast cancersurvivors. BMC Cancer. 2012;12(1):1–10. [PMC free article] [PubMed]5.
Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008;14(1):14–24. [PubMed]6.
Montazeri A, Vahdaninia M, Harirchi I, Ebrahimi M, Khaleghi F, Jarvandi S. Quality of life in patients with breast cancer before and after diagnosis: an eighteen months follow-up study. BMC Cancer. 2008;8:330. [PMC free article] [PubMed]7.
Roundtree AK, Giordano SH, Price A, Suarez-Almazor ME. Problems in transition and quality of care: perspectives of breast cancer survivors. Support Care Cancer. 2011;19(12):1921–1929. [PMC free article] [PubMed]8.
Nesvold IL, Reinertsen KV, Fossa SD, Dahl AA. The relation between arm/shoulder problems and quality of life in breast cancer survivors: a cross-sectional and longitudinal study. J Cancer Surviv. 2011;5(1):62–72. [PMC free article] [PubMed]9.
Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J Clin Oncol. 2005;23(10):2378–2388. [PubMed]10.
Ligibel JA, Meyerhardt J, Pierce JP, et al. Impact of a telephone-based physical activity intervention upon exercise behaviors and fitness in cancer survivors enrolled in a cooperative group setting. Breast Cancer Res. 2012;132(1):205–213. [PMC free article] [PubMed]11.
Holick CN, Newcomb PA, Trentham-Dietz A, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(2):379–386. [PubMed]12.
Basen-Engquist K, Hughes D, Perkins H, Shinn E, Taylor CC. Dimensions of physical activity and their relationship to physical and emotional symptoms in breast cancer survivors. J Cancer Surviv. 2008;2(4):253–261. [PMC free article] [PubMed]13.
Ogunleye AA, Holmes MD. Physical activity and breast cancer survival. Breast Cancer Res. 2009;11(5):106. [PMC free article] [PubMed]14.
Mandelblatt JS, Luta G, Kwan ML, et al. Associations of physical activity with quality of life and functional ability in breast cancer patients during active adjuvant treatment: the pathways study. Breast Cancer Res Treat. 2011;129(2):521–529. [PMC free article] [PubMed]15.
Paxton RJ, Phillips KL, Jones LA, et al. Associations among physical activity, body mass index, and health-related quality of life by race/ethnicity in a diverse sample of breast cancer survivors. Cancer. 2012;118(16):4024–4031. [PMC free article] [PubMed]16.
Vallance JK, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol. 2007;25(17):2352–2359. [PubMed]17.
Dimeo FC, Thomas F, Raabe-Menssen C, Propper F, Mathias M. Effect of aerobic exercise and relaxation training on fatigue and physical performance of cancer patients after surgery: a randomised controlled trial. Support Care Cancer. 2004;12(11):774–779. [PubMed]18.
Kim CJ. A meta-analysis of aerobic exercise interventions for women with breast cancer. West J Nurs Res. 2009;31(4):437–461. [PubMed]19.
McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175(1):34–41. [PMC free article] [PubMed]20.
Floyd A, Moyer A. Group vs individual exercise interventions for women with breast cancer: a meta-analysis. Health Psychol Rev. 2009;4(1):22–41. [PMC free article] [PubMed]21.
Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2015;15:77. [PMC free article] [PubMed]22.
Pan Y, Yang K, Shi X, Liang H, Zhang F, Lv Q. Tai chi chuan exercise for patients with breast cancer: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2015;2015:535237. [PMC free article] [PubMed]23.
Verhagen AP, de Vet HC, de Bie RA, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–1241. [PubMed]24.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [PMC free article] [PubMed]25.
Hayashino J, Noguchi Y, Fukui T. Systematic evaluation and comparison of statistical tests for publication bias. J Epidemiol. 2005;15(6):235–243. [PubMed]26.
Basen-Engquist K, Taylor CL, Rosenblum C, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Educ Couns. 2006;64(1–3):225–234. [PubMed]27.
Battaglini C. The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. Sao Paulo Med J. 2007;125(1):22–28. [PubMed]28.
Bower JE, Greendale G, Crosswell AD, et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. 2014;43:20–29. [PMC free article] [PubMed]29. Cantarero-Villanueva I, Fernández-Lao C, Díaz-Rodriguez L, Fernández-de-las-Peñas C, del Moral-Avila R, Arroyo-Morales M. A multimodal exercise program and multimedia support reduce cancer-related fatigue in breast cancer survivors: a randomised controlled clinical trial. Eur J Integr Med. 2011;3(3):e189–e200.
30.
Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396–4404. [PubMed]31.
Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25(13):1713–1721. [PubMed]32.
Drouin JS, Young TJ, Beeler J, et al. Random control clinical trial on the effects of aerobic exercise training on erythrocyte levels during radiation treatment for breast cancer. Cancer. 2006;107(10):2490–2495. [PubMed]33.
Irwin ML, Alvarez-Reeves M, Cadmus L, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity. 2009;17(8):1534–1541. [PMC free article] [PubMed]34.
Irwin ML, Varma K, Alvarez-Reeves M, et al. Randomized controlled trial of aerobic exercise on insulin and insulin-like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):306–313. [PMC free article] [PubMed]35.
Janelsins MC, Davis PG, Wideman L, et al. Effects of Tai Chi Chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clin Breast Cancer. 2011;11(3):161–170. [PMC free article] [PubMed]36.
Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008;108(2):279–288. [PubMed]37.
Mutrieet N, Campbell AM, Whyte F, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ. 2007;334(7592):517. [PMC free article] [PubMed]38.
Nikander R, Sievanen H, Ojala K, Oivanen T, Kellokumpu-Lehtinen PL, Saarto T. Effect of a vigorous aerobic regimen on physical performance in breast cancer patients – a randomized controlled pilot trial. Acta Oncol. 2007;46(2):181–186. [PubMed]39.
Rao MR, Raghuram N, Nagendra HR, et al. Anxiolytic effects of a yoga program in early breast cancer patients undergoing conventional treatment: a randomized controlled trial. Complement Ther Med. 2009;17(1):1–8. [PubMed]40.
Rogers LQ, Fogleman A, Trammell R, et al. Effects of a physical activity behavior change intervention on inflammation and related health outcomes in breast cancer survivors: pilot randomized trial. Integr Cancer Ther. 2013;12(4):323–335. [PMC free article] [PubMed]41.
Rogers LQ, Hopkins-Price P, Vicari S, et al. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41(4):935–946. [PubMed]42.
Vadiraja HS, Rao MR, Nagarathna R, et al. Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Complement Ther Med. 2009;17(5–6):274–280. [PubMed]43.
Battaglini CL. Effect of exercise on the caloric intake of breast cancer patients undergoing treatment. Braz J Med Biol Res. 2008;41:709–715. [PubMed]44.
Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660–1668. [PubMed]45.
Hwang JH, Chang HJ, Shim YH, et al. Effects of supervised exercise therapy in patients receiving radiotherapy for breast cancer. Yonsei Med J. 2008;49(3):443–450. [PMC free article] [PubMed]46.
Sandel SL, Judge JO, Landry N, Faria L, Ouellette R, Majczak M. Dance and movement program improves quality-of-life measures in breast cancer survivors. Cancer Nurs. 2005;28:301–309. [PubMed]47.
Moadel AB, Shah C, Wylie-Rosett J, et al. Randomized controlled trial of yoga among a multiethnic sample of breast cancer patients: effects on quality of life. J Clin Oncol. 2007;25(28):4387–4395. [PubMed]48.
Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2003;12:721–727. [PubMed]49.
Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis protein. Cancer Epidemiol Biomarkers Prev. 2005;14:1672–1680. [PubMed]50.
Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118(15):3766–3775. [PMC free article] [PubMed]51.
Cadmus LA, Salovey P, Yu H, Chung G, Kasl S, Irwin ML. Exercise and quality of life during and after treatment for breast cancer: results of two randomized controlled trials. Psychooncology. 2009;18(4):343–352. [PMC free article] [PubMed]52.
Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psychooncology. 2009;18(4):360–368. [PMC free article] [PubMed]53.
Hayes SC, Rye S, Disipio T, et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat. 2013;137(1):175–186. [PubMed]54.
Littman AJ, Bertram LC, Ceballos R, et al. Randomized controlled pilot trial of yoga in overweight and obese breast cancer survivors: effects on quality of life and anthropometric measures. Support Care Cancer. 2012;20(2):267–277. [PMC free article] [PubMed]55.
Mustian KM, Katula JA, Gill DL, Roscoe JA, Lang D, Murphy K. Tai Chi Chuan, health-related quality of life and self-esteem: a randomized trial with breast cancer survivors. Support Care Cancer. 2004;12(12):871–876. [PubMed]56.
Ohira T, Schmitz KH, Ahmed RL, Yee D. Effects of weight training on quality of life in recent breast cancer survivors: the Weight Training for Breast Cancer Survivors (WTBS) study. Cancer. 2006;106(9):2076–2083. [PubMed]57.
Sprod LK, Janelsins MC, Palesh OG, et al. Health-related quality of life and biomarkers in breast cancer survivors participating in tai chi chuan. J Cancer Surviv. 2012;6(2):146–154. [PMC free article] [PubMed]58.
Saarto T, Penttinen HM, Sievannen H. Effectiveness of a 12-month exercise program on physical performance and quality of life of breast cancer survivors. Anticancer Res. 2012;32:3875–3884. [PubMed]59.
Duijts SF, Faber MM, Oldenburg HS, van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors – a meta-analysis. Psychooncology. 2011;20(2):115–126. [PubMed]60.
Ferrer RA, Huedo-Medina TB, Johnson BT, Ryan S, Pescatello LS. Exercise interventions for cancer survivors: a meta-analysis of quality of life outcomes. Ann Behav Med. 2011;41(1):32–47. [PMC free article] [PubMed]61.
Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. [PMC free article] [PubMed]62.
Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. [PubMed]63.
Schmitz KH, Holtzman J, Courneya KS, Mâsse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1588–1595. [PubMed]64.
Brown JC, Huedo-Medina TB, Pescatello LS, et al. The efficacy of exercise in reducing depressive symptoms among cancer survivors: a meta-analysis. PLoS One. 2012;7(1):e30955. [PMC free article] [PubMed]65.
Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferreer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Am Association Cancer Res. 2010;10(1158):1055–9965. [PubMed]66.
Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: a systematic review. Can Treat Rev. 2010;36(2):185–194. [PubMed]67.
Buffart LM, van Uffelen JG, Riphagen II. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2012;12:559. [PMC free article] [PubMed]68.
Nichols HB, Trentham-Dietz A, Egan KM, et al. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1403–1409. [PMC free article] [PubMed]69.
Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20(1):42–51. [PubMed]70.
Kleinberg DL, Wood TL, Furth PA, Lee AV. Growth hormone and insulin-like growth factor-I in the transition from normal mammary development to preneoplastic mammary lesions. Endocr Rev. 2009;30(1):51–74. [PubMed]71.
Bertram LA, Stefanick ML, Saquib N, et al. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: findings from the WHEL Study. Cancer Causes Control. 2011;22(3):427–435. [PMC free article] [PubMed]72.
Sternfeld B, Weltzien E, Quesenberry CP, et al. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18(1):87–95. [PMC free article] [PubMed]73.
Adamsen L, Quist M, Andersen C, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ. 2009;339:b3410. [PMC free article] [PubMed]74.
Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol. 2006;24(18):2765–2772. [PubMed]75.
Andrykowski MA, Beacham AO, Jacobsen PB. Prospective, longitudinal study of leisure-time exercise in women with early-stage breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(3):430–438. [PubMed]76.
Kwan ML, Sternfeld B, Ergas IJ, et al. Change in physical activity during active treatment in a prospective study of breast cancer survivors. Breast Cancer Res Treat. 2012;131(2):679–690. [PMC free article] [PubMed]77.
Valenti M, Porzio G, Aielli F, et al. Physical exercise and quality of life in breast cancer survivors. Inter J Med Sci. 2008;5(1):24–28. [PMC free article] [PubMed]
Articles from OncoTargets and therapy are provided here courtesy of Dove Press
lichaamsbeweging, borstkanker, chemo, radiotherapie, hormoontherapie, kwaliteit van leven, overall overleving, kans op overlijden, vermoeidheid, sporten
Gerelateerde artikelen



 1
1
Plaats een reactie ...
Reageer op "Fysieke activiteit van borstkankerpatienten na 1 jaar nog in leven na behandeling heeft grote invloed - 35 tot 52 procent - op kans op overlijden"