1 november 2018: Lees ook dit artikel:
30 mei 2016: Lees ook dit artikel:
30 mei 2016: Inmiddels is het volledige studierapport van onderstaande studie gratis in te zien. Phase I/II Study of Oncolytic HSVGM-CSF in Combination with Radiotherapy and Cisplatin in Untreated Stage III/IV Squamous Cell Cancer of the Head and Neck
Eindconclusie blijft staan:
JS1/34.5-/47-/GM-CSF gecombineerd met cisplatin en chemoradiotherapie wordt als veilig en goed te tolereren ervaren door patienten met vergevorderde hoofd- en halstumoren die vooraf al chemo hebben gehad op platinumbasis. De aanbevolen dosis uit de fase II studie is 10(6), 10(8), 10(8), 10(8). Locoregionale controle werd bereikt bij alle patienten met een 76.5% recidiefvrije tijd tot zover. Ik kan nog steeds geen recentere resultaten vinden maar die zullen er ongetwijfeld zijn. Al is de studie: Study of Safety and Efficacy of Talimogene Laherparepvec With Cisplatin and Radiotherapy for Treatment of Locally Advanced Head and Neck Cancer om onbekende redenen stopgezet. Maar ik neem toch aan dat de resultaten uit de fase II studie jaren later wel bekend zullen zijn. Het is natuurlijk wel vreemd dat zo'n succesvolle studie zomaar wordt stopgezet. En tegelijkertijd studies bij andere vormen van kanker met dezelfde aanpak worden voortgezet of nieuw opgezet.
Ik heb het bedrijf dat Oncovec (Talimogene laherparepvec) produceert hierover een mailtje gestuurd. Overigens wordt immuuntherapie met dit virus bij meerdere vormen van kanker onderzocht, o.a. bij melanomen en borstkanker
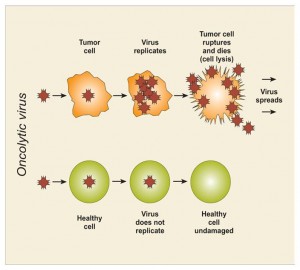
Hoe werkt dit Oncolytisch virus met de naam Oncovex GM-GSF (Talimogene laherparepvec)? In Wikipedia staat m.i. interessante informatie over dit virus:
https://en.wikipedia.org/wiki/Talimogene_laherparepvec#Efficacy_in_head_and_neck_cancer
Hier enkele grafieken uit dat studierapport.
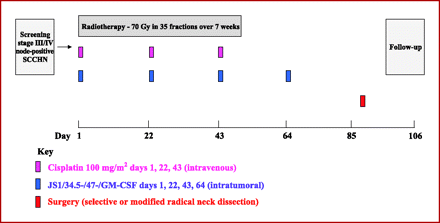
Fig. 1.
Treatment schedule for cisplatin-based chemoradiation and concomitant intratumoral injections of JS1/34.5-/47-/GM-CSF, followed by planned neck dissection. Patients received full-dose radical chemoradiotherapy comprising 70 Gy in 35 daily fractions given 5 days per week (Monday to Friday) over 7 weeks with cisplatin given on days 1, 22, and 43 at a standard dose (100 mg/m2 body surface area). Virus was injected on days 1, 22, 43, and 64 (this last dose was administered 2 weeks after the end of CRT). Injected volumes were based on the following algorithm: 0.5 mL for nodes of 0.5 to 1.5 cm longest dimension; 1 mL for nodes of 1.6 to 2.5 cm longest dimension; 2 mL for nodes >2.5 cm longest dimension. The maximum virus dose delivered on any one treatment day was 4 mL. Patients were scheduled to undergo resection of involved cervical lymph nodes 6 to 8 weeks after the end of CRT.

Fig. 2.
Q-PCR from tumor-containing lymph node tissue showing viral recovery in seven patients. Note that patient 001-0006 did not provide a presurgery or surgical biopsy specimen. Patient study numbers and the cohort in which they were treated are indicated. HSV detection is expressed as genome copy number per gram of tumor tissue (see text for details). The number above each bar represents the interval (in days) between the last injection of JS1/34.5-/47-/GM-CSF and sample collection.
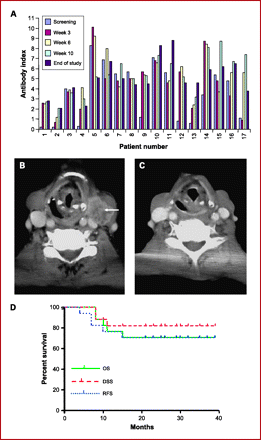
Fig. 4.
A, neutralizing antibody response to JS1/34.5-/47-/GM-CSF. Data are expressed in terms of the antibody index (AI): seronegative = AI <0.9; seropositive = AI >1.1; equivocal = AI 0.9-1.1. Seven patients were seronegative at the start of treatment, all of whom seroconverted during treatment. There was a dose-dependent increase in mean (SD) AI: cohort 1, 2.85 (0.54); cohort 2, 5.53 (0.58); cohort 3, 6.49 (1.60). B and C, pretreatment and posttreatment CT images of patient 001-0007 in cohort 2. The large left level 3 nodal mass (arrow) seen pretreatment (B) completely resolved posttreatment (C). D. Kaplan-Meier survival curves showing overall (OS), disease-specific (DSS) and progression-free survival (PFS).
Immuuntherapie met het oncolytisch virus geeft de volgende bijwerkingen die allemaal mild en goed te behandelen zijn:
Adverse events of any grade reported in ≥10% of patients during study treatment regardless of causality
| Adverse event | JS1/34.5-/47-/GM-CSF dose group | All patients (n = 17) | |||
|---|---|---|---|---|---|
| Cohort 1 (n = 4) | Cohort 2 (n = 4) | Cohort 3 (n = 4) | Cohort 4 (n = 5) | ||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Any event | 4 (100) | 4 (100) | 4 (100) | 5 (100) | 17 (100) |
| Weight decreased | 3 (75) | 4 (100) | 4 (100) | 5 (100) | 16 (94) |
| Constipation | 4 (100) | 3 (75) | 3 (75) | 5 (100) | 15 (88) |
| Mucosal inflammation | 3 (75) | 4 (100) | 3 (75) | 4 (80) | 14 (82) |
| Radiation skin injury | 1 (25) | 4 (100) | 4 (100) | 5 (100) | 14 (82) |
| Anemia | 2 (50) | 4 (100) | 4 (100) | 3 (60) | 13 (76) |
| Nausea | 4 (100) | 4 (100) | 2 (50) | 3 (60) | 13 (76) |
| Dysphagia | 4 (100) | 3 (75) | 1 (25) | 4 (80) | 12 (71) |
| Pyrexia | 3 (75) | 2 (50) | 1 (25) | 4 (80) | 10 (59) |
| Dehydration | 1 (25) | 1 (25) | 4 (100) | 3 (60) | 9 (53) |
| Dry mouth | 0 (0) | 3 (75) | 2 (50) | 4 (80) | 9 (53) |
| Neutropenia | 2 (50) | 3 (75) | 2 (50) | 1 (20) | 8 (47) |
| Pharyngolaryngeal pain | 2 (50) | 2 (50) | 1 (25) | 3 (60) | 8 (47) |
| Leukopenia | 1 (25) | 4 (100) | 1 (25) | 1 (20) | 7 (41) |
| Fatigue | 1 (25) | 0 (0) | 2 (50) | 3 (60) | 6 (35) |
| Odynophagia | 1 (25) | 2 (50) | 2 (50) | 1 (20) | 6 (35) |
| Oral candidiasis | 0 (0) | 0 (0) | 3 (75) | 3 (60) | 6 (35) |
| Vomiting | 2 (50) | 1 (25) | 1 (25) | 2 (40) | 6 (35) |
| Anorexia | 1 (25) | 0 (0) | 2 (50) | 2 (40) | 5 (29) |
| Diarrhea | 3 (75) | 1 (25) | 0 (0) | 1 (20) | 5 (29) |
| Tinnitus | 1 (25) | 0 (0) | 2 (50) | 2 (40) | 5 (29) |
| Dysgeusia | 0 (0) | 0 (0) | 2 (50) | 2 (40) | 4 (24) |
| Insomnia | 2 (50) | 0 (0) | 1 (25) | 1 (20) | 4 (24) |
| Mouth ulceration | 0 (0) | 1 (25) | 1 (25) | 2 (40) | 4 (24) |
| Renal failure | 1 (25) | 2 (50) | 1 (25) | 0 (0) | 4 (24) |
| Anxiety | 2 (50) | 0 (0) | 0 (0) | 1 (20) | 3 (18) |
| Confusional state | 0 (0) | 2 (50) | 1 (25) | 0 (0) | 3 (18) |
| Cough | 1 (25) | 0 (0) | 1 (25) | 1 (20) | 3 (18) |
| Depressed mood | 1 (25) | 0 (0) | 1 (25) | 1 (20) | 3 (18) |
| Dyspepsia | 2 (50) | 0 (0) | 0 (0) | 1 (20) | 3 (18) |
| Febrile neutropenia | 2 (50) | 0 (0) | 1 (25) | 0 (0) | 3 (18) |
| Hypokalemia | 0 (0) | 1 (25) | 1 (25) | 1 (20) | 3 (18) |
| Hyponatremia | 1 (25) | 1 (25) | 1 (25) | 0 (0) | 3 (18) |
| Lymphopenia | 1 (25) | 1 (25) | 0 (0) | 1 (20) | 3 (18) |
| Rash | 0 (0) | 0 (0) | 3 (75) | 0 (0) | 3 (18) |
| Stomatitis | 1 (25) | 0 (0) | 1 (25) | 1 (20) | 3 (18) |
| Thrombocytopenia | 0 (0) | 1 (25) | 2 (50) | 0 (0) | 3 (18) |
| Abdominal discomfort | 0 (0) | 0 (0) | 1 (25) | 1 (20) | 2 (12) |
| Azotemia | 1 (25) | 1 (25) | 0 (0) | 0 (0) | 2 (12) |
| Depression | 0 (0) | 2 (50) | 0 (0) | 0 (0) | 2 (12) |
| Dizziness | 0 (0) | 0 (0) | 2 (50) | 0 (0) | 2 (12) |
| Dysphonia | 0 (0) | 2 (50) | 0 (0) | 0 (0) | 2 (12) |
| Erythema | 1 (25) | 0 (0) | 1 (25) | 0 (0) | 2 (12) |
| Headache | 0 (0) | 1 (25) | 0 (0) | 1 (20) | 2 (12) |
| Hypogeusia | 0 (0) | 0 (0) | 1 (25) | 1 (20) | 2 (12) |
| Hypotension | 0 (0) | 1 (25) | 1 (25) | 0 (0) | 2 (12) |
| Injection site hemorrhage | 0 (0) | 0 (0) | 1 (25) | 1 (20) | 2 (12) |
| Injection site reaction | 0 (0) | 0 (0) | 1 (25) | 1 (20) | 2 (12) |
| Lower respiratory tract infection | 1 (25) | 1 (25) | 0 (0) | 0 (0) | 2 (12) |
| Malaise | 2 (50) | 0 (0) | 0 (0) | 0 (0) | 2 (12) |
| Pharyngitis | 1 (25) | 1 (25) | 0 (0) | 0 (0) | 2 (12) |
| Stomach discomfort | 0 (0) | 0 (0) | 1 (25) | 1 (20) | 2 (12) |
| Syncope | 1 (25) | 0 (0) | 1 (25) | 0 (0) | 2 (12) |
NOTE: Cohort 1, 106 pfu/mL on four occasions, maximum of 4 mL per dose. Cohort 2, 106 pfu/mL once followed by 107 on three occasions, maximum of 4 mL per dose. Cohort 3, 106 pfu/mL once followed by 108 on three occasions, maximum of 4 mL per dose. Expansion cohort, 106 pfu/mL once followed by 108 on three occasions, maximum of 8 mL per dose.
Ondraan artikel staat nog een referentielisjt die hoort bij deze studie, lees verder onderstaand dat ik al in 2010 plaatste.
2 augustus 2010: Bron: Clin Cancer Res. 2010 Aug 1;16(15):4005-15.
Injecties met het herpes virus naast chemo voorkomt recidief bij patienten met een operabele mond- en keelkanker stadium III/IV. Dit blijkt uit een kleinschalige fase I/II studie met 17 patienten. Het resultaat is significant. Na een follow-up van 29 maanden bleek 82,4% van de deelnemende patienten nog steeds ziektevrij. Met alleen chemo ligt dat percentage historisch gezien tussen de 40 en 45%. Zo goed als een verdubbeling van het resultaat. Hier het abstract zoals dat in Clinical Cancer research is gepubliceerd:
Clin Cancer Res. 2010 Aug 1;16(15):4005-15.
Phase I/II Study of Oncolytic HSVGM-CSF in Combination with Radiotherapy and Cisplatin in Untreated Stage III/IV Squamous Cell Cancer of the Head and Neck.
Harrington KJ, Hingorani M, Tanay MA, Hickey J, Bhide SA, Clarke PM, Renouf LC, Thway K, Sibtain A, McNeish IA, Newbold KL, Goldsweig H, Coffin R, Nutting CM.
Authors' Affiliations: The Institute of Cancer Research, The Royal Marsden Hospital, St Bartholomew's Hospital, and Barts and The London School of Medicine, London, United Kingdom; and BioVex Inc, Woburn, Massachusetts.
Abstract
PURPOSE: This study sought to define the recommended dose of JS1/34.5-/47-/GM-CSF, an oncolytic herpes simplex type-1 virus (HSV-1) encoding human granulocyte-macrophage colony-stimulating factor (GM-CSF), for future studies in combination with chemoradiotherapy in patients with squamous cell cancer of the head and neck (SCCHN).
EXPERIMENTAL DESIGN: Patients with stage III/IVA/IVB SCCHN received chemoradiotherapy (70 Gy/35 fractions with concomitant cisplatin 100 mg/m(2) on days 1, 22, and 43) and dose-escalating (10(6), 10(6), 10(6), 10(6) pfu/mL for cohort 1; 10(6), 10(7), 10(7), 10(7) for cohort 2; 10(6), 10(8), 10(8), 10(8) for cohort 3) JS1/34.5-/47-/GM-CSF by intratumoral injection on days 1, 22, 43, and 64. Patients underwent neck dissection 6 to 10 weeks later. Primary end points were safety and recommended dose/schedule for future study. Secondary end points included antitumor activity (radiologic, pathologic). Relapse rates and survival were also monitored. Results: Seventeen patients were treated without delays to chemoradiotherapy or dose-limiting toxicity. Fourteen patients (82.3%) showed tumor response by Response Evaluation Criteria in Solid Tumors, and pathologic complete remission was confirmed in 93% of patients at neck dissection. HSV was detected in injected and adjacent uninjected tumors at levels higher than the input dose, indicating viral replication. All patients were seropositive at the end of treatment. No patient developed locoregional recurrence, and disease-specific survival was 82.4% at a median follow-up of 29 months (range, 19-40 months).
CONCLUSIONS: JS1/34.5-/47-/GM-CSF combined with cisplatin-based chemoradiotherapy is well tolerated in patients with SCCHN. The recommended phase II dose is 10(6), 10(8), 10(8), 10(8). Locoregional control was achieved in all patients, with a 76.5% relapse-free rate so far. Further study of this approach is warranted in locally advanced SCCHN. Clin Cancer Res; 16(15); 4005-15. (c)2010 AACR.
PMID: 20670951 [PubMed - in process]
References
Gerelateerde artikelen



Plaats een reactie ...
Reageer op "Virussen: Injecties met een herpes virus na operatie van tumoren in mond en keel en gegeven naast chemo voorkomt significant recidief. Artikel geplaatst 2 augustus 2010"