En zie in gerelateerde artikelen meer publicaties over maretakinjecties.
22 november 2018: Het volledige studierapport van onderstaand beschreven studie:
Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: A randomised clinical trial on overall survival is inmiddels gratis in te zien.
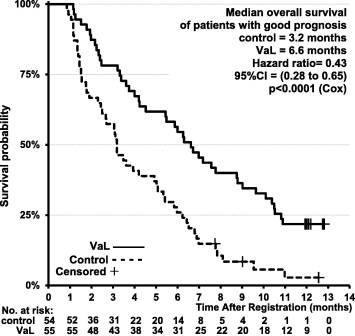
Hier de grafiek van de overall overleving van alle 220 patiënten. (zie verder studierapprot of ons artikel uit 2013 hieronder).

Fig. 5
Forest plot (multivariate cox regression including interactions) of the treatment effect on 12-month overall survival of 220 patients with advanced or metastatic pancreatic cancer assigned to a therapy with extract of VaL or to no antineoplastic therapy (Control). Abbreviations: ECOG, Eastern Cooperative Oncology Group (performance scale); VaL, Viscum album [L.]. The squares represent the hazard ratio and their sizes are proportional to the sizes of the subgroups. The horizontal lines show the confidence intervals.
Ik heb de referentielijst behorend bij deze studie onderaan dit artikel ook toegevoegd.

7 december 2014:het volledige studierapport van onderstaande studie: Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: A randomised clinical trial on overall survival is inmiddels gratis in te zien. Zie hieronder abstract en vertaalde studieresultaten. Zie ook uitgewerkte grafieken voor patiënten met goede prognose en met slechte prognose, in beide groepen bleek de maretakinjecties mediaan significant betere overleving te geven. Al was dat soms maar enkele maanden. Hoe goed zou het zijn om hiermee te starten bij patienten die nog in goede conditie zijn, waarbij de tumor zoveel mogelijk is weggehaald enz.
En aanvullend op onderstaande publicatie is aan de hand van dezelfde studie een studie gepubliceerd die laat zien dat maretakinjecties de kwaliteit van leven significant verbetert. Het volledige studierapport: Quality of Life of Patients With Advanced Pancreatic Cancer During Treatment With Mistletoe is gratis in te zien. Het abstract heb ik onderaan toegevoegd.
6 september 2013: Meer informatie over maretakinjecties, inclusief een voorlichtingsvideo staat onder complementair - maretakinjecties
30 augustus 2013: Bron: Eur J Cancer. 2013 Jul 24. pii: S0959-8049(13)00550-9. doi: 10.1016/j.ejca.2013.06.043. [Epub ahead of print].
Viscum Album L. - Maretakinjecties verdubbelen mediane overleving bij inoperabele uitgezaaide alvleesklierkankerpatiënten in vergelijking met beste zorg. Dit blijkt uit een gerandomiseerde fase III studie bij totaal 220 patiënten waarvoor geen adekwate behandeling meer mogelijk is. De studie maakte een vergelijking tussen beste zorg en beste zorg plus Viscum album L. subcutaan - onderhuids gegeven. Ter informatie: intraveneus gegeven lijkt beter te werken dan onderhuids aldus enkele artsen die ik daarover heb gesproken, maar blijkbaar werkt onderhuids gegeven ook.
De gegevens uit het abstract zijn echter dermate summier dat ik niet zo heel veel meer aan informatie kan geven. Hier de zo goed als letterlijke vertaling van het abstract. Het volledige studierapport: Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: A randomised clinical trial on overall survival is gratis in te zien. Onderaan originele engelstalige abstract. Hier de letterlijke vertaling met hulp van google translation van dat abstract:
ACHTERGROND:
De ongunstige neveneffecten van het behandelen van laat-stadiumalvleesklierkanker vragen om niet-toxische en effectievere therapeutische benaderingen. We vergeleken de totale overleving (OS) van de patiënten die een extract van Viscum album [L.] (Val) kregen of geen antineoplastische therapie (red: geen chemo of andere behandeling).
Methode:
Dit is een prospectieve, parallelle, open-label, monocenter, groeps-sequentiële, gerandomiseerde fase III studie. Patiënten met lokaal gevorderde of uitgezaaide kanker van de pancreas - alvleesklier werden gestratificeerd naar een binaire prognose index, samengesteld uit tumorstadium, leeftijd en performance status, en werden gelijkmatig gerandomiseerd ingedeeld naar subcutane injecties van Viscum album L. (VAL) extracten of geen antineoplastische therapie (controlegroep). VaL werd toegepast in een dosis opgevoerde wijze van 0,01 mg tot 10 mg driemaal per week. Patiënten in beide groepen kregen de beste ondersteunende zorg. Het primaire eindpunt was 12 maanden OS, beoordeeld in een groep-sequentiële analyse.
BEVINDINGEN:
We presenteren de eerste tussentijdse analyse, met inbegrip van gegevens van 220 patiënten. Baseline karakteristieken waren evenwichtig verdeeld tussen de studiegroepen. Mediane overleving - OS was 4.8 maanden voor de Viscum album L. (VAL) groep en 2.7 maanden voor de controlegroep. (prognose gecorrigeerde hazard ratio, HR = 0,49, p <0,0001). Binnen de zogeheten 'goede' prognose subgroep, mediane OS was 6,6 maanden versus 3.2 maanden (HR = 0,43, p <0,0001). Binnen de 'slechte' prognose subgroep was dit 3,4 maanden versus 2.0 maanden respectievelijk (HR = 0,55, p = 0,0031). er werden geen aan Viscum album L. (VAL) gerelateerde bijwerkingen waargenomen.
CONCLUSIE:
Viscum album L. (VAL) injecties toonden een significante en klinisch relevante verlenging van de mediane overleving - OS. De onderzoeksresultaten suggereren dat Viscum album L. (VAL) een niet-toxisch en effectieve tweedelijns therapie kan zijn die een verlenging van de overleving - OS en minder ziekte gerelateerde symptomen kan opleveren voor patiënten met inoperabele, lokaal gevorderde of uitgezaaide alvleesklierkanker.
Het volledige studierapport: Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: A randomised clinical trial on overall survival is inmiddels gratis in te zien.
Hier het originele engelstalige abstract:
Viscum Album L. therapy showed a significant and clinically relevant prolongation of overall survival - OS. The study findings suggest Viscum Album L. - VaL (mistletoe injections) to be a non-toxic and effective second-line therapy that offers a prolongation of overall survival - OS as well as less disease-related symptoms for patients with locally advanced or metastatic pancreatic cancer.
Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: A randomised clinical trial on overall survival.
Source
Clinical Research Dr. Tröger, Freiburg, Germany. Electronic address: troeger@crdt.de.
Abstract
BACKGROUND:
The unfavourable side-effects of late-stage pancreatic cancer treatments call for non-toxic and effective therapeutic approaches. We compared the overall survival (OS) of patients receiving an extract of Viscum album [L.] (VaL) or no antineoplastic therapy.
METHODS:
This is a prospective, parallel, open label, monocentre, group-sequential, randomised phase III study. Patients with locally advanced or metastatic cancer of the pancreas were stratified according to a binary prognosis index, composed of tumour stage, age and performance status; and were evenly randomised to subcutaneous injections of VaL extracts or no antineoplastic therapy (control). VaL was applied in a dose-escalating manner from 0.01mg up to 10mg three times per week. Patients in both groups received best supportive care. The primary end-point was 12-month OS, assessed in a group-sequential analysis.
FINDINGS:
We present the first interim analysis, including data from 220 patients. Baseline characteristics were well balanced between the study arms. Median OS was 4.8 for VaL and 2.7months for control patients (prognosis-adjusted hazard ratio, HR=0.49; p<0.0001). Within the 'good' prognosis subgroup, median OS was 6.6 versus 3.2months (HR=0.43; p<0.0001), within the 'poor' prognosis subgroup, it was 3.4 versus 2.0months respectively (HR=0.55; p=0.0031). No VaL-related adverse events were observed.
CONCLUSION:
VaL therapy showed a significant and clinically relevant prolongation of OS. The study findings suggest VaL to be a non-toxic and effective second-line therapy that offers a prolongation of OS as well as less disease-related symptoms for patients with locally advanced or metastatic pancreatic cancer.
Copyright © 2013 The Authors. Published by Elsevier Ltd.. All rights reserved.
In patients with locally advanced or metastatic pancreatic carcinoma, mistletoe treatment significantly improves the quality of life in comparison to best supportive care alone. Mistletoe is an effective second-line treatment for this disease.
Quality of Life of Patients With Advanced Pancreatic Cancer During Treatment With Mistletoe
Abstract
Background
The treatment of cancer patients with mistletoe extract is said to prolong their survival and, above all, improve their quality of life. We studied whether the quality of life of patients with advanced pancreatic cancer could be improved by mistletoe extract.
Method
An open, single-center, group-sequential, randomized phase III trial (ISRCTN70760582) was conducted. From January 2009 to December 2010, 220 patients with locally advanced or metastatic pancreatic cancer who were receiving no further treatment for pancreatic cancer other than best supportive care were included in this trial. They were stratified by prognosis and randomly allocated either to a group that received mistletoe treatment or to one that did not. Mistletoe extract was given in escalating doses by subcutaneous injection three times a week. The planned interim evaluation of data from 220 patients indicated that mistletoe treatment was associated with longer overall survival, and the trial was terminated prematurely. After termination of the study, the results with respect to quality of life (assessed with the QLO-C30 scales of the European Organisation for Research and Treatment of Cancer) and trends in body weight were evaluated.
Results
Data on quality of life and body weight were obtained from 96 patients treated with mistletoe and 72 control patients. Those treated with mistletoe did better on all 6 functional scales and on 7 of 9 symptom scales, including pain (95% confidence interval -29 to –17), fatigue (95% CI –36.1 to –25.0), appetite loss (95% CI -51 to -36.7), and insomnia (95% CI –45.8 to –28.6). This is reflected by the trend in body weight during the trial.
Conclusion
In patients with locally advanced or metastatic pancreatic carcinoma, mistletoe treatment significantly improves the quality of life in comparison to best supportive care alone. Mistletoe is an effective second-line treatment for this disease.
Referentielijst studie maretak injecties bij alvleesklierkanker
References
- 1Bayraktar, S., Bayraktar, U.D., and Rocha-Lima, C.M. Recent developments in palliative chemotherapy for locally advanced and metastatic pancreas cancer. World J Gastroenterol. 2010; 16: 673–682
- 2Cascinu, S., Falconi, M., Valentini, V., and Jelic, S. Pancreatic cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010; 21: v55–v58
- 3Conroy, T., Desseigne, F., Ychou, M. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011; 364: 1817–1825
- 4Richter, J. and Saif, M.W. Locally advanced pancreatic adenocarcinoma: where are we and where are we going? Highlights from the “2010 ASCO Gastrointestinal Cancers Symposium”. Orlando, FL, USA. January 22–24, 2010. JOP. 2010 Mar 5; 11: 139–143
- 5Jacobs, A.D., Burris, H.A. III, Rivkin, S. et al. A randomized phase III study of rubitecan (ORA) vs. best choice (BC) in 409 patients with refractory pancreatic cancer report from a North-American multi-center study. J Clin Oncol. 2004; 22: 4013
- 6Pelzer, U., Schwaner, I., Stieler, J. et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011; 47: 1676–1681
- 7Boeck, S., Bruns, C.J., Sargent, M. et al. Current oncological treatment of patients with pancreatic cancer in germany: results from a national survey on behalf of the Arbeitsgemeinschaft Internistische Onkologie and the Chirurgische Arbeitsgemeinschaft Onkologie of the Germany Cancer Society. Oncology. 2009; 77: 40–48
- 8Cragg, G.M. and Newman, D.J. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005; 100: 72–79
- 9Kienle, G.S. and Kiene, H. Review article: influence of Viscum album L (European mistletoe) extracts on quality of life in cancer patients: a systematic review of controlled clinical studies. Integr Cancer Ther. 2010; 9: 142–157
- 10Büssing, A. Mistletoe. The genus Viscum. (p. 1–265)Hardwood Academic Publishers, Amsterdam; 2000
- 11Büssing, A., Wagner, M., Wagner, B. et al. Induction of mitochondrial Apo2.7 molecules and generation of reactive oxygen-intermediates in cultured lymphocytes by the toxic proteins from Viscum album L. Cancer Lett. 1999; 139: 79–88
- 12Kienle, G.S. and Kiene, H. Die Mistel in der onkologie – fakten und konzeptionelle grundlagen. (p. 1–749)Schattauer Verlag, Stuttgart; 2003
- 13Thies, A., Dautel, P., Meyer, A., Pfuller, U., and Schumacher, U. Low-dose mistletoe lectin-I reduces melanoma growth and spread in a scid mouse xenograft model. Br J Cancer. 2008; 98: 106–112
- 14Rostock, M., Huber, R., Greiner, T. et al. Anticancer activity of a lectin-rich mistletoe extract injected intratumorally into human pancreatic cancer xenografts. Anticancer Res. 2005; 25: 1969–1975
- 15Matthes, H., Buchwald, D., Schad, F., and Jeschke, E. Intratumorale applikation von Viscum album L. (Mistelgesamtextrakt; Helixor M) in der therapie des inoperablen pankreaskarzinom. (http://dx.doi.org/10.1055/s-2007-988162)Z Gastroenterol. 2007; 45
- 16Mansky, P.J., Blackman, M.R., Grem, J., Swain, S.M., and Monahan, B.P. NCCM/NCI phase I study of mistletoe extract and gemcitabine in patients with advanced solid tumors. J Clin Oncol. 2010; 28: 2559
- 17Kienle, G.S., Grugel, R., and Kiene, H. Safety of higher dosages of Viscum album L. in animals and humans – systematic review of immune changes and safety parameters. BMC Complement Altern Med. 2011; 11: 72
- 18Rostock, M. and Huber, R. Randomized and double-blind studies – demands and reality as demonstrated by two examples of mistletoe research. Forsch Komplementärmed Klass Naturheilkd. 2004; 11: 18–22
- 19Guidance for Industry. Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071590.pdf 2007 May.
- 20Matthes, H., Friedel, W.E., Bock, P.R., and Zanker, K.S. Molecular mistletoe therapy: friend or foe in established anti-tumor protocols? A multicenter, controlled, retrospective pharmaco-epidemiological study in pancreas cancer. Curr Mol Med. 2010; 10: 430–439
- 21Schaefermeyer, G. and Schaefermeyer, H. Treatment of pancreatic cancer with Viscum album (Iscador): a retrospective study of 292 patients 1986–1996. Complement Ther Med. 1998; 6: 172–177
- 22Freedman, L.S. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982; 1: 121–129
- 23Lan, K.K. and DeMets, D.L. Discrete sequential boundaries for clinical trials. Biometrika. 1983; 70: 659–663
- 24Emerson, S.S. and Fleming, T.R. Parameter estimation following group sequential hypothesis testing. Biometrika. 1990; 77: 875–892
- 25Hrobjartsson, A. and Gotzsche, P.C. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010; 20: CD003974
- 26Grossarth-Maticek, R. and Ziegler, R. Randomised and non-randomised prospective controlled cohort studies in matched-pair design for the long-term therapy of breast cancer patients with a mistletoe preparation (Iscador): a re-analysis. Eur J Med Res. 2006; 11: 485–495
- 27Kienle, G.S., Berrino, F., Büssing, A. et al. Mistletoe in cancer – a systematic review on controlled clinical trials. Eur J Med Res. 2003; 8: 109–119
- 28Kienle, G.S. and Kiene, H. Systematic reviews on mistletoe in cancer – what implications for future research can be drawn?. Phytomedicine (Jena). 2007; 14: 11
- 29Kleeberg, U.R., Suciu, S., Bröcker, E.B. et al. Final results of the EORTC 18871/DKG 80–1 randomised phase III trial: rIFN-a2b versus rIFN-g versus Iscador M versus observation after surgery in melanoma patients with either high-risk primary (thickness >3 mm) or regional lymph node metastasis. Eur J Cancer. 2004; 40: 390–402
- 30Steuer-Vogt, M.K., Bonkowsky, V., Ambrosch, P. et al. The effect of an adjuvant mistletoe treatment programme in resected head and neck cancer patients: a randomised controlled clinical trial. Eur J Cancer. 2001; 37: 23–31
- 31Fung, M.C., Takayama, S., Ishiguro, H. et al. Chemotherapy for advanced or metastatic pancreatic cancer: analysis of 43 randomized trials in 3 decades (1974–2002). Gan To Kagaku Ryoho. 2003; 30: 1101–1111
- 32Andersen, J.R., Friis-Moller, A., Hancke, S. et al. A controlled trial of combination chemotherapy with 5-FU and BCNU in pancreatic cancer. Scand J Gastroenterol. 1981; 16: 973–975
- 33Andren-Sandberg, A., Holmberg, J.T., and Ihse, I. Treatment of unresectable pancreatic carcinoma with 5-fluorouracil, vincristine, and CCNU. Scand J Gastroenterol. 1983; 18: 609–612
- 34Ciuleanu, T.E., Pavlovsky, A.V., Bodoky, G. et al. A randomised phase III trial of glufosfamide compared with best supportive care in metastatic pancreatic adenocarcinoma previously treated with gemcitabine. Eur J Cancer. 2009; 45: 1589–1596
- 35Gilliam, A.D., Broome, P., Topuzov, E.G. et al. An international multicenter randomized controlled trial of G17DT in patients with pancreatic cancer. Pancreas. 2012; 41: 374–379
- 36Glimelius, B., Hoffman, K., Sjoden, P.O. et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996; 7: 593–600
- 37Huguier, M., Barrier, A., Valinas, R. et al. Randomized trial of 5-fluorouracil, leucovorin and cisplatin in advanced pancreatic cancer. Hepatogastroenterology. 2001; 48: 875–878
- 38Koeppler, H., Duru, M., Grundheber, M. et al. Palliative treatment of advanced pancreatic carcinoma in community-based oncology group practices. J Support Oncol. 2004; 2: 159–163
- 39Mallinson, C.N., Rake, M.O., Cocking, J.B. et al. Chemotherapy in pancreatic cancer: results of a controlled, prospective, randomised, multicentre trial. Br Med J. 1980; 281: 1589–1591
- 40Matsumoto, K., Miyake, Y., Kato, H. et al. Effect of low-dose gemcitabine on unresectable pancreatic cancer in elderly patients. Digestion. 2011; 84: 230–235
- 41Negi, S.S., Agarwal, A., and Chaudhary, A. Flutamide in unresectable pancreatic adenocarcinoma: a randomized, double-blind, placebo-controlled trial. Invest New Drugs. 2006; 24: 189–194
- 42Palmer, K.R., Kerr, M., Knowles, G. et al. Chemotherapy prolongs survival in inoperable pancreatic carcinoma. Br J Surg. 1994; 81: 882–885
- 43Rosenberg, L., Barkun, A.N., Denis, M.H., and Pollak, M. Low dose octreotide and tamoxifen in the treatment of adenocarcinoma of the pancreas. Cancer. 1995; 75: 23–28
- 44Shimoda, M., Katoh, M., Kita, J., Sawada, T., and Kubota, K. The Glasgow Prognostic Score is a good predictor of treatment outcome in patients with unresectable pancreatic cancer. Chemotherapy. 2010; 56: 501–506
- 45Shinchi, H., Takao, S., Noma, H. et al. Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2002; 53: 146–150
- 46Takada, T., Nimura, Y., Katoh, H. et al. Prospective randomized trial of 5-fluorouracil, doxorubicin, and mitomycin C for non-resectable pancreatic and biliary carcinoma: multicenter randomized trial. Hepatogastroenterology. 1998; 45: 2020–2026
- 47Taylor, O.M., Benson, E.A., and McMahon, M.J. Clinical trial of tamoxifen in patients with irresectable pancreatic adenocarcinoma. The Yorkshire Gastrointestinal Tumour Group. Br J Surg. 1993; 80: 384–386
- 48Tsavaris, N., Tentas, K., Tzivras, M. et al. Combined epirubicin, 5-fluorouracil and folinic acid vs no treatment for patients with advanced pancreatic cancer: a prospective comparative study. J Chemother. 1998; 10: 331–337
- 49Wang, P., Meng, Z.Q., Chen, Z. et al. Survival rate of pancreatic cancer in elderly patients. Hepatogastroenterology. 2008; 55: 681–686
- 50Weinerman, B.H. and MacCormick, R.E. A phase II survival comparison of patients with adenocarcinoma of the pancreas treated with 5-fluorouracil and calcium leucovorin versus a matched tumor registry control population. Am J Clin Oncol. 1994; 17: 467–469
Plaats een reactie ...
4 Reacties op "Maretakinjecties - Viscum album L. verdubbelt mediane overleving bij uitgezaaide, inoperabele alvleesklierkanker met betere kwaliteit van leven"
Gerelateerde artikelen
- Matters studie onderzoekt effecten van hyperthermie bij kankerpatienten met solide tumoren en patienten met alvleesklierkanker.
- Studiepublicaties van voeding, voedingstoffen, niet-toxische middelen en behandelingen uit literatuurlijst van arts-bioloog drs. Engelbert Valstar, specifiek bij alvleesklierkanker
- Enzymtherapie: Studierapport van Dr. Gonzalez en zijn aanpak met pancreasenzymen bij alvleesklierkankerpatiënten. Met opmerkelijk positieve uitkomsten.
- Hyperthermie bij alvleesklierkanker: aantal studies laten zien dat hyperthermie naast bestraling en chemo een aanzienlijk beter effect geeft op overall overlevingstijd.
- Maretakinjecties - Viscum album L. verdubbelt mediane overleving bij uitgezaaide, inoperabele alvleesklierkanker met betere kwaliteit van leven
- Voeding en voedingsstoffen: Bepaalde vorm van vitamine D - 1a,25(OH)2D ((Paricalcitol) voorkomt en geeft positief effect in behandelen van alvleesklierkanker, blijkt uit overzichtstudie.
- Complementair - niet-toxisch - aanvullend bij alvleesklierkanker: een overzicht van artikelen en studie resultaten met niet toxische behandelingen, voedingstoffen en andere middelen als aanvulling of als mono behandeling van alvleesklierkanker



Voor Joyce: ik zou contact opnemen met prof. dr. Casper van Eijck - Erasmus Rotterdam of misschein nog VUmc Amsterdam Interventieradiologie?
Kees Braam
webmaster www.kanker-actueel.nl
e-mail: redactie@kanker-actueel.nl