13 augustus 2021: zie ook dit artikel: https://kanker-actueel.nl/olaparib-een-parpremmer-gegeven-aan-borstkankerpatienten-met-brca-1-en-brca-2-na-operatie-en-chemotherapie-verbetert-ziektevrije-overleving-met-9-procent.html
31 december 2016: Bronnen: clinical trials, NPO, NKI
Vandaag in het NPO nieuws minister Schippers die meedeelt dat een experimentele therapie voor borstkanker met zogeheten BRCA-like status voorlopig vergoed gaat worden uit de basisverzekering. Hier wat meer infomatie over deze vorm van behandelen en de studie waar het omgaat. Volgens dr. Sabine Linn stijgt met deze aanpak de 10-jaarsoverleving naar 70% tot 80%, aangetoond in drie onafhankelijk van elkaar uitgevoerde fase II studies.
31 december 2016: Vooraf mijn commentaar: is dit een verkapte regeringssteun aan de farmacie? Is dit handjeklap met de ziektekostenverzekeraars en oneigenlijk gebruik maken van haar positie wat Minister Schippers doet? Want betalen voor een placebo gecontroleerde studie betekent dat niet iedere patient uit deze studie hiervan profiteert. En in geval van vergoedingen vanuit de basisverzekering, die toch voor alle borstkankerpatienten met die specifieke mutatie zou moeten gelden al helemaal niet want alleen de patienten die meedoen aan de studie krijgen dit vergoed. Alle andere borstkankerpatienten met BRCA-like mutatie dus niet. Ik heb hier grote vraagtekens bij. Want gaat ten koste van de premies voor de ziektekostenverzekeringen m.i.. Maar voor betrokken patienten is het natuurlijk wel goed nieuws.
Als eerste zoals dit bericht wordt gemeld op de website van het NKI waar deze studie wordt uitgevoerd:
Experimentele behandeling erfelijke borstkanker in basispakket
29dec 2016

De NOS berichtte vandaag, donderdag 29 december, dat een experimentele behandeling bij erfelijke borstkanker en hieraan verwante borstkanker in het basispakket komt van de zorgverzekering. Het is een behandeling voor vrouwen met BRCA1-like, stadium III borstkanker. Vrouwen die in aanmerking komen krijgen een hoge dosis chemotherapie en stamceltransplantatie.
Vanaf komende zondag zit deze behandeling in het basispakket, heeft minister Schippers besloten. Omdat het een experimentele behandeling is, wordt die voorwaardelijk tot het basispakket toegelaten. 'De kans is groot dat deze experimentele behandeling uiteindelijk voorgoed tot het basispakket wordt toegelaten omdat verschillende terugkijkende studies een positief resultaat laten zien: van de patiënten die een standaard behandeling krijgen is 30 tot 40% van de patiënten ziektevrij na 10 jaar. Van de patiënten die deze experimentele behandeling hebben ondergaan is dat 78%', aldus prof. dr. Sabine Linn, internist-oncoloog in het Antoni van Leeuwenhoek. Lees verder het hele artikel>>>>>>>>>>
In het NPO journaal (29 december 2016 om 18.00 uur en 20.00 uur) wordt dr. Sabine Linn geïnterviewd en het valt mij op hoe ongelukkig zij zich voelt dat deze studie een placebo gecopntroleerde studie is. Zij weet heel goed dat de helft van de deelnemende patienten dus niet deze experimentele behandeling krijgen, terwijl ze daarmee dus de kans op overall overleven zouden kunnen verdubbelen van 30% tot 40% naar 70 tot 80%. Dit zou toch anders moeten kunnen zie je haar bijna hardop denken.
Hier vindt u het studieprotocol van deze studie:
Substantially improving the cure rate of high-risk BRCA1-like breast cancer patients with personalized therapy
Uw behandelend arts kan hier informatie vragen en eventueel aanmelden:
B.5.1
|
Name of organisation |
NKI-AVL |
| B.5.2 |
Functional name of contact point |
Study Coordinator |
| B.5.3 |
Address: |
| B.5.3.1 |
Street Address |
Plesmanlaan 121 |
| B.5.3.2 |
Town/ city |
Amsterdam |
| B.5.3.3 |
Post code |
1066CX |
| B.5.3.4 |
Country |
Netherlands |
| B.5.6 |
E-mail |
s.vliek@nki.nl |
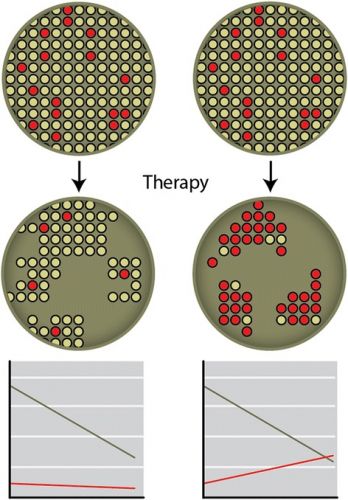
Tekst gaat verder onder dit beeld:
Bron foto: Cytotoxic and targeted therapy for hereditary cancers
Een interessant studierapport in dit verband is dit volledige studierapport:
gratis in te zien met interessante referentielijst, zie onderaan dit artikel:
Aan dit artikel is vele uren gewerkt. Opzoeken, vertalen, op de website plaatsen enz. Als u ons wilt ondersteunen dan kan dat via een al of niet anonieme donatie. Elk bedrag is welkom hoe klein ook. Klik hier als u ons wilt helpen kanker-actueel online te houden Wij zijn een ANBI organisatie en dus is uw donatie in principe aftrekbaar voor de belasting
Borstkanker stamcel onderzoek
Interessant over deze vorm van behandelen met stamcellen staat op deze Amerikaanse website met enkele video's waarin uitgelegd wordt hoe gewerkt wordt met stamcellen bij borstkanker en ook waarom deze zo goed werken:
Breast Cancer Stem Cell Research
In the fight against breast cancer, there is good news and bad news. The good news is that, since 1990, there has been a steady decline in the death rate from breast cancer. Earlier detection and better treatments are bringing hope to people with both early and advanced disease.
The bad news is that more than 40,000 people die from breast cancer every year in the United States alone. It is still the second-leading cause of deaths from cancer in women. The survival rate for those with advanced, metastatic breast cancer has not changed significantly for decades. In spite of more effective therapies, many patients still experience recurrences of breast cancer after treatment. Read more and watch the video's>>>>>>>>>>
Cytotoxic and targeted therapy for hereditary cancers
Cytotoxic and targeted therapy for hereditary cancers
Abstract
There is a number of drugs demonstrating specific activity towards hereditary cancers. For example, tumors in BRCA1/2 mutation carriers usually arise via somatic inactivation of the remaining BRCA allele, which makes them particularly sensitive to platinum-based drugs, PARP inhibitors (PARPi), mitomycin C, liposomal doxorubicin, etc. There are several molecular assays for BRCA-ness, which permit to reveal BRCA-like phenocopies among sporadic tumors and thus extend clinical indications for the use of BRCA-specific therapies. Retrospective data on high-dose chemotherapy deserve consideration given some unexpected instances of cure from metastatic disease among BRCA1/2-mutated patients. Hereditary non-polyposis colorectal cancer (HNPCC) is characterized by high-level microsatellite instability (MSI-H), increased antigenicity and elevated expression of immunosuppressive molecules. Recent clinical trial demonstrated tumor responses in HNPCC patients treated by the immune checkpoint inhibitor pembrolizumab. There are successful clinical trials on the use of novel targeted agents for the treatment or rare cancer syndromes, e.g. RET inhibitors for hereditary medullary thyroid cancer, mTOR inhibitors for tumors arising in patients with tuberous sclerosis (TSC), and SMO inhibitors for basal-cell nevus syndrome. Germ-line mutation tests will be increasingly used in the future for the choice of the optimal therapy, therefore turnaround time for these laboratory procedures needs to be significantly reduced to ensure proper treatment planning.
Conclusions and perspectives
The number of known hereditary cancer syndromes will rapidly grow within next several years, thanks to the invention of whole exome sequencing [131]. Many of already identified hereditary cancer types are represented by exceptionally rare instances of the disease, and future investigations are likely to reveal even more uncommon cancer syndromes. In addition, unlike many other medical conditions, genetic diseases are often population-specific, i.e. their spread is limited by a few ethnic groups. It is unrealistic to expect that each hereditary cancer type will be subjected to systematic laboratory investigations and comprehensive clinical trials. There are some approaches which may facilitate search for novel treatment strategies for orphan and/or hereditary cancer types. For instance, collection and analysis of biological material from these patients deserve to be encouraged. In addition, some ex vivo testing for tumor drug sensitivity may turn out to be particularly practical in this clinical setting [156]. There are also some bioinformatic tools pretending to predict drug sensitivity of the tumor based on its molecular characteristics [157]. Finally, several potentially practice-changing investigations in this field became possible due to availability of large clinical databases [16, 27, 120]. Well-designed retrospective studies may help to significantly improve the use of existing cancer therapies.
References
1.
Malkin D, Li FP, Strong LC, Fraumeni JF, Jr, Nelson CE, Kim DH, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [PubMed] [Cross Ref]2.
Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, et al. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [PubMed] [Cross Ref]3.
Leach FS, Nicolaides NC, Papadopoulos N, Liu B, Jen J, Parsons R, et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993;75:1215–1225. doi: 10.1016/0092-8674(93)90330-S. [PubMed] [Cross Ref]4.
Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [PubMed] [Cross Ref]5.
Wooster R, Neuhausen SL, Mangion J, Quirk Y, Ford D, Collins N, et al. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994;265:2088–2090. doi: 10.1126/science.8091231. [PubMed] [Cross Ref]6.
Imyanitov EN, Moiseyenko VM. Drug therapy for hereditary cancers. Hered Cancer Clin Pract. 2011;9(1):5. doi: 10.1186/1897-4287-9-5. [PMC free article] [PubMed] [Cross Ref]7.
Bayraktar S, Glück S. Systemic therapy options in BRCA mutation-associated breast cancer. Breast Cancer Res Treat. 2012;135(2):355–366. doi: 10.1007/s10549-012-2158-6. [PubMed] [Cross Ref]8.
Maxwell KN, Domchek SM. Cancer treatment according to BRCA1 and BRCA2 mutations. Nat Rev Clin Oncol. 2012;9(9):520–528. doi: 10.1038/nrclinonc.2012.123. [PubMed] [Cross Ref]9.
Imyanitov EN, Byrski T. Systemic treatment for hereditary cancers: a 2012 update. Hered Cancer Clin Pract. 2013;11(1):2. doi: 10.1186/1897-4287-11-2. [PMC free article] [PubMed] [Cross Ref]10.
Turner NC, Tutt AN. Platinum chemotherapy for BRCA1-related breast cancer: do we need more evidence? Breast Cancer Res. 2012;14(6):115. doi: 10.1186/bcr3332. [PMC free article] [PubMed] [Cross Ref]11.
Pothuri B. BRCA1- and BRCA2-related mutations: therapeutic implications in ovarian cancer. Ann Oncol. 2013;24(Suppl 8):viii22–viii27. doi: 10.1093/annonc/mdt307. [PubMed] [Cross Ref]12.
Lee JM, Ledermann JA, Kohn EC. PARP Inhibitors for BRCA1/2 mutation-associated and BRCA-like malignancies. Ann Oncol. 2014;25(1):32–40. doi: 10.1093/annonc/mdt384. [PMC free article] [PubMed] [Cross Ref]13.
Sonnenblick A, de Azambuja E, Azim HA, Jr, Piccart M. An update on PARP inhibitors--moving to the adjuvant setting. Nat Rev Clin Oncol. 2015;12(1):27–41. doi: 10.1038/nrclinonc.2014.163. [PubMed] [Cross Ref]14.
Byrski T, Huzarski T, Dent R, Gronwald J, Zuziak D, Cybulski C, et al. Response to neoadjuvant therapy with cisplatin in B RCA1-positive breast cancer patients. Breast Cancer Res Treat. 2009;115(2):359–363. doi: 10.1007/s10549-008-0128-9. [PubMed] [Cross Ref]15.
Byrski T, Huzarski T, Dent R, Marczyk E, Jasiowka M, Gronwald J, et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147(2):401–405. doi: 10.1007/s10549-014-3100-x. [PubMed] [Cross Ref]16.
Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28(3):375–379. doi: 10.1200/JCO.2008.20.7019. [PubMed] [Cross Ref]17.
Pfeifer W, Sokolenko AP, Potapova ON, Bessonov AA, Ivantsov AO, Laptiev SA, et al. Breast cancer sensitivity to neoadjuvant therapy in BRCA1 and CHEK2 mutation carriers and non-carriers. Breast Cancer Res Treat. 2014;148(3):675–683. doi: 10.1007/s10549-014-3206-1. [PubMed] [Cross Ref]18.
Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28(7):1145–1153. doi: 10.1200/JCO.2009.22.4725. [PMC free article] [PubMed] [Cross Ref]19.
Kołacińska A, Chałubińska J, Błasińska-Morawiec M, Dowgier-Witczak I, Fendler W, Kordek R, et al. Pathological complete response in younger and older breast cancer patients. Arch Med Sci. 2012;8(2):310–315. doi: 10.5114/aoms.2012.28559. [PMC free article] [PubMed] [Cross Ref]20.
Moiseyenko VM, Dolmatov GD, Moiseyenko FV, Ivantsov AO, Volkov NM, Chubenko VA, et al. High efficacy of cisplatin neoadjuvant therapy in a prospective series of patients carrying BRCA1 germ-line mutation. Med Oncol. 2015;32(4):89. doi: 10.1007/s12032-015-0514-1. [PubMed] [Cross Ref]21.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [PubMed] [Cross Ref]22.
Byrski T, Dent R, Blecharz P, Foszczynska-Kloda M, Gronwald J, Huzarski T, et al. Results of a phase II open-label, non-randomized trial of cisplatin chemotherapy in patients with BRCA1-positive metastatic breast cancer. Breast Cancer Res. 2012;14(4):R110. doi: 10.1186/bcr3231. [PMC free article] [PubMed] [Cross Ref]23.
Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, et al. TBCRC009: A Multicenter Phase II Clinical Trial of Platinum Monotherapy With Biomarker Assessment in Metastatic Triple-Negative Breast Cancer. J Clin Oncol. 2015;33(17):1902–1909. doi: 10.1200/JCO.2014.57.6660. [PMC free article] [PubMed] [Cross Ref]24. Tutt A, Ellis P, Kilburn L, Gilett C, Pinder S, Abraham J, et al. The TNT trial: A randomized phase III trial of carboplatin (C) compared with docetaxel (D) for patients with metastatic or recurrent locally advanced triple negative or BRCA1/2 breast cancer (CRUK/07/012). San Antonio Breast Cancer Symposium. 2014:abstr S3-01.
25.
Lord CJ, Ashworth A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat Med. 2013;19(11):1381–1388. doi: 10.1038/nm.3369. [PubMed] [Cross Ref]26.
Chalasani P, Livingston R. Differential chemotherapeutic sensitivity for breast tumors with “BRCAness”: a review. Oncologist. 2013;18(8):909–916. doi: 10.1634/theoncologist.2013-0039. [PMC free article] [PubMed] [Cross Ref]27.
Vencken PM, Kriege M, Hoogwerf D, Beugelink S, van der Burg ME, Hooning MJ, et al. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol. 2011;22(6):1346–1352. doi: 10.1093/annonc/mdq628. [PubMed] [Cross Ref]28.
Alsop K, Fereday S, Meldrum C, de Fazio A, Emmanuel C, George J, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30(21):2654–2663. doi: 10.1200/JCO.2011.39.8545. [PMC free article] [PubMed] [Cross Ref]29.
Gorodnova TV, Sokolenko AP, Ivantsov AO, Iyevleva AG, Suspitsin EN, Aleksakhina SN, et al. High response rates to neoadjuvant platinum-based therapy in ovarian cancer patients carrying germ-line BRCA mutation. Cancer Lett. 2015;369(2):363–367. doi: 10.1016/j.canlet.2015.08.028. [PubMed] [Cross Ref]30.
Mahdi H, Gockley A, Esselen K, Marquard J, Nutter B, Yang B, et al. Outcome of neoadjuvant chemotherapy in BRCA1/2 mutation positive women with advanced-stage Müllerian cancer. Gynecol Oncol. 2015;139(3):407–412. doi: 10.1016/j.ygyno.2015.07.101. [PubMed] [Cross Ref]31.
Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [PubMed] [Cross Ref]32.
Patel AG, De Lorenzo SB, Flatten KS, Poirier GG, Kaufmann SH. Failure of iniparib to inhibit poly(ADP-Ribose) polymerase in vitro. Clin Cancer Res. 2012;18(6):1655–1662. doi: 10.1158/1078-0432.CCR-11-2890. [PMC free article] [PubMed] [Cross Ref]33.
Mateo J, Ong M, Tan DS, Gonzalez MA, de Bono JS. Appraising iniparib, the PARP inhibitor that never was--what must we learn? Nat Rev Clin Oncol. 2013;10(12):688–696. doi: 10.1038/nrclinonc.2013.177. [PubMed] [Cross Ref]34.
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–134. doi: 10.1056/NEJMoa0900212. [PubMed] [Cross Ref]35.
Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28(15):2512–2519. doi: 10.1200/JCO.2009.26.9589. [PubMed] [Cross Ref]36.
Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–244. doi: 10.1016/S0140-6736(10)60892-6. [PubMed] [Cross Ref]37.
Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–251. doi: 10.1016/S0140-6736(10)60893-8. [PubMed] [Cross Ref]38.
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–250. doi: 10.1200/JCO.2014.56.2728. [PubMed] [Cross Ref]39.
van der Noll R, Marchetti S, Steeghs N, Beijnen JH, Mergui-Roelvink MW, Harms E, et al. Long-term safety and anti-tumour activity of olaparib monotherapy after combination with carboplatin and paclitaxel in patients with advanced breast, ovarian or fallopian tube cancer. Br J Cancer. 2015;113(3):396–402. doi: 10.1038/bjc.2015.256. [PMC free article] [PubMed] [Cross Ref]40.
Domchek SM, Aghajanian C, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140(2):199–203. doi: 10.1016/j.ygyno.2015.12.020. [PMC free article] [PubMed] [Cross Ref]41.
Matulonis UA, Penson RT, Domchek SM, Kaufman B, Shapira-Frommer R, Audeh MW, et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: a multi-study analysis of response rates and safety. Ann Oncol. 2016 (in press). [PubMed]42.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomized phase 2 trial. Lancet Oncol. 2014;15(8):852–861. doi: 10.1016/S1470-2045(14)70228-1. [PubMed] [Cross Ref]43.
Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A. 2008;105(44):17079–17084. doi: 10.1073/pnas.0806092105. [PMC free article] [PubMed] [Cross Ref]44.
Karginova O, Siegel MB, Van Swearingen AE, Deal AM, Adamo B, Sambade MJ, et al. Efficacy of Carboplatin Alone and in Combination with ABT888 in Intracranial Murine Models of BRCA-Mutated and BRCA-Wild-Type Triple-Negative Breast Cancer. Mol Cancer Ther. 2015;14(4):920–930. doi: 10.1158/1535-7163.MCT-14-0474. [PMC free article] [PubMed] [Cross Ref]45.
Ávila-Arroyo S, Nuñez GS, García-Fernández LF, Galmarini CM. Synergistic Effect of Trabectedin and Olaparib Combination Regimen in Breast Cancer Cell Lines. J Breast Cancer. 2015;18(4):329–338. doi: 10.4048/jbc.2015.18.4.329. [PMC free article] [PubMed] [Cross Ref]46.
Lee JM, Hays JL, Annunziata CM, Noonan AM, Minasian L, Zujewski JA, et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J Natl Cancer Inst. 2014;106(6):dju089. doi: 10.1093/jnci/dju089. [PMC free article] [PubMed] [Cross Ref]47.
Del Conte G, Sessa C, von Moos R, Viganò L, Digena T, Locatelli A, et al. Phase I study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumours. Br J Cancer. 2014;111(4):651–659. doi: 10.1038/bjc.2014.345. [PMC free article] [PubMed] [Cross Ref]48.
Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RH, Sonke GS, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomized phase 2 trial. Lancet Oncol. 2015;16(1):87–97. doi: 10.1016/S1470-2045(14)71135-0. [PubMed] [Cross Ref]49.
Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30(4):372–379. doi: 10.1200/JCO.2011.36.9215. [PubMed] [Cross Ref]50.
Konstantinopoulos PA, Cannistra SA. Comparing poly (ADP-ribose) polymerase inhibitors with standard chemotherapy in BRCA-mutated, recurrent ovarian cancer: lessons learned from a negative trial. J Clin Oncol. 2012;30(4):347–350. doi: 10.1200/JCO.2011.40.1489. [PubMed] [Cross Ref]51.
Coleman RL, Sill MW, Bell-McGuinn K, Aghajanian C, Gray HJ, Tewari KS, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation - An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2015;137(3):386–391. doi: 10.1016/j.ygyno.2015.03.042. [PMC free article] [PubMed] [Cross Ref]52.
Sandhu SK, Schelman WR, Wilding G, Moreno V, Baird RD, Miranda S, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14(9):882–892. doi: 10.1016/S1470-2045(13)70240-7. [PubMed] [Cross Ref]53.
Drew Y, Ledermann J, Hall G, Rea D, Glasspool R, Highley M, et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br J Cancer. 2016;114(7):723–730. doi: 10.1038/bjc.2016.41. [PMC free article] [PubMed] [Cross Ref]54.
Moiseyenko VM, Chubenko VA, Moiseyenko FV, Zhabina AS, Gorodnova TV, Komarov YI, et al. Evidence for clinical efficacy of mitomycin C in heavily pretreated ovarian cancer patients carrying germ-line BRCA1 mutation. Med Oncol. 2014;31(10):199. doi: 10.1007/s12032-014-0199-x. [PubMed] [Cross Ref]55.
D’Incalci M. Trabectedin mechanism of action: what’s new? Future Oncol. 2013;9(12 Suppl):5–10. doi: 10.2217/fon.13.207. [PubMed] [Cross Ref]56.
Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, et al. Trabectedin plus pegylated liposomal Doxorubicin in recurrent ovarian cancer. J Clin Oncol. 2010;28(19):3107–3114. doi: 10.1200/JCO.2009.25.4037. [PubMed] [Cross Ref]57.
Delaloge S, Wolp-Diniz R, Byrski T, Blum JL, Gonçalves A, Campone M, et al. Activity of trabectedin in germline BRCA1/2-mutated metastatic breast cancer: results of an international first-in-class phase II study. Ann Oncol. 2014;25(6):1152–1158. doi: 10.1093/annonc/mdu134. [PubMed] [Cross Ref]58.
Lorusso D, Scambia G, Pignata S, Sorio R, Amadio G, Lepori S, et al. Prospective phase II trial of trabectedin in BRCA-mutated and/or BRCAness phenotype recurrent ovarian cancer patients: the MITO 15 trial. Ann Oncol. 2016;27(3):487–493. doi: 10.1093/annonc/mdv608. [PubMed] [Cross Ref]59.
Kaklamani VG, Jeruss JS, Hughes E, Siziopikou K, Timms KM, Gutin A, et al. Phase II neoadjuvant clinical trial of carboplatin and eribulin in women with triple negative early-stage breast cancer (NCT01372579) Breast Cancer Res Treat. 2015;151(3):629–638. doi: 10.1007/s10549-015-3435-y. [PubMed] [Cross Ref]60.
Kriege M, Jager A, Hooning MJ, Huijskens E, Blom J, van Deurzen CH, et al. The efficacy of taxane chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer. 2012;118(4):899–907. doi: 10.1002/cncr.26351. [PubMed] [Cross Ref]61.
Arun B, Bayraktar S, Liu DD, Gutierrez Barrera AM, Atchley D, Pusztai L, et al. Response to neoadjuvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol. 2011;29(28):3739–3746. doi: 10.1200/JCO.2011.35.2682. [PMC free article] [PubMed] [Cross Ref]62.
Tan DS, Yap TA, Hutka M, Roxburgh P, Ang J, Banerjee S, et al. Implications of BRCA1 and BRCA2 mutations for the efficacy of paclitaxel monotherapy in advanced ovarian cancer. Eur J Cancer. 2013;49(6):1246–1253. doi: 10.1016/j.ejca.2012.11.016. [PubMed] [Cross Ref]63.
Burness ML, Obeid EI, Olopade OI. Triple negative breast cancer in BRCA1 mutation carriers with a complete radiologic response to neoadjuvant paclitaxel: a case report. Clin Breast Cancer. 2015;15(2):e155–e158. doi: 10.1016/j.clbc.2014.08.006. [PubMed] [Cross Ref]64.
King TA, Li W, Brogi E, Yee CJ, Gemignani ML, Olvera N, et al. Heterogenic loss of the wild-type BRCA allele in human breast tumorigenesis. Ann Surg Oncol. 2007;14(9):2510–2518. doi: 10.1245/s10434-007-9372-1. [PubMed] [Cross Ref]65.
Curtit E, Benhamo V, Gruel N, Popova T, Manie E, Cottu P, et al. First description of a sporadic breast cancer in a woman with BRCA1 germline mutation. Oncotarget. 2015;6(34):35616–35624. [PMC free article] [PubMed]66.
Safra T, Borgato L, Nicoletto MO, Rolnitzky L, Pelles-Avraham S, Geva R, et al. BRCA mutation status and determinant of outcome in women with recurrent epithelial ovarian cancer treated with pegylated liposomal doxorubicin. Mol Cancer Ther. 2011;10(10):2000–2007. doi: 10.1158/1535-7163.MCT-11-0272. [PubMed] [Cross Ref]67.
Safra T, Rogowski O, Muggia FM. The effect of germ-line BRCA mutations on response to chemotherapy and outcome of recurrent ovarian cancer. Int J Gynecol Cancer. 2014;24(3):488–495. doi: 10.1097/IGC.0000000000000086. [PubMed] [Cross Ref]68.
Hamad L, Khoury T, Vona K, Nestico J, Opyrchal M, Salerno KE. A Case of Metaplastic Breast Cancer with Prolonged Response to Single Agent Liposomal Doxorubicin. Cureus. 2016;8(1):e454. [PMC free article] [PubMed]69.
Kummar S, Oza AM, Fleming GF, Sullivan DM, Gandara DR, Naughton MJ, et al. Randomized Trial of Oral Cyclophosphamide and Veliparib in High-Grade Serous Ovarian, Primary Peritoneal, or Fallopian Tube Cancers, or BRCA-Mutant Ovarian Cancer. Clin Cancer Res. 2015;21(7):1574–1582. doi: 10.1158/1078-0432.CCR-14-2565. [PMC free article] [PubMed] [Cross Ref]70.
De P, Sun Y, Carlson JH, Friedman LS, Leyland-Jones BR, Dey N. Doubling down on the PI3K-AKT-mTOR pathway enhances the antitumor efficacy of PARP inhibitor in triple negative breast cancer model beyond BRCA-ness. Neoplasia. 2014;16(1):43–72. doi: 10.1593/neo.131694. [PMC free article] [PubMed] [Cross Ref]71.
Mo W, Liu Q, Lin CC, Dai H, Peng Y, Liang Y, et al. mTOR Inhibitors Suppress Homologous Recombination Repair and Synergize with PARP Inhibitors via Regulating SUV39H1 in BRCA-Proficient Triple-Negative Breast Cancer. Clin Cancer Res. 2016;22(7):1699–1712. doi: 10.1158/1078-0432.CCR-15-1772. [PMC free article] [PubMed] [Cross Ref]72.
Ibrahim YH, García-García C, Serra V, He L, Torres-Lockhart K, Prat A, et al. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012;2(11):1036–1047. doi: 10.1158/2159-8290.CD-11-0348. [PMC free article] [PubMed] [Cross Ref]73.
Juvekar A, Burga LN, Hu H, Lunsford EP, Ibrahim YH, Balmañà J, et al. Combining a PI3K inhibitor with a PARP inhibitor provides an effective therapy for BRCA1-related breast cancer. Cancer Discov. 2012;2(11):1048–1063. doi: 10.1158/2159-8290.CD-11-0336. [PMC free article] [PubMed] [Cross Ref]74.
Wang D, Wang M, Jiang N, Zhang Y, Bian X, Wang X, et al. Effective use of PI3K inhibitor BKM120 and PARP inhibitor Olaparib to treat PIK3CA mutant ovarian cancer. Oncotarget. 2016;7((11):13153–13166. [PMC free article] [PubMed]75.
Jóhannsson OT, Idvall I, Anderson C, Borg A, Barkardóttir RB, Egilsson V, et al. Tumour biological features of BRCA1-induced breast and ovarian cancer. Eur J Cancer. 1997;33(3):362–371. doi: 10.1016/S0959-8049(97)89007-7. [PubMed] [Cross Ref]76.
Strickland KC, Howitt BE, Shukla SA, Rodig S, Ritterhouse LL, Liu JF, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016 (in press). doi:10.18632/oncotarget.7277. [PMC free article] [PubMed]77.
Vesprini D, Narod SA, Trachtenberg J, Crook J, Jalali F, Preiner J, et al. The therapeutic ratio is preserved for radiotherapy or cisplatin treatment in BRCA2-mutated prostate cancers. Can Urol Assoc J. 2011;5(2):E31–E35. doi: 10.5489/cuaj.10080. [PMC free article] [PubMed] [Cross Ref]78.
Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373(18):1697–1708. doi: 10.1056/NEJMoa1506859. [PubMed] [Cross Ref]79.
Chedgy EC, Annala M, Beja K, Warner EW, Gleave ME, Chi KN, et al. Moving Toward Personalized Care: Liquid Biopsy Predicts Response to Cisplatin in an Unusual Case of BRCA2-Null Neuroendocrine Prostate Cancer. Clin Genitourin Cancer. 2016;14(2):e233–e236. doi: 10.1016/j.clgc.2015.12.023. [PubMed] [Cross Ref]80.
Luo G, Lu Y, Jin K, Cheng H, Guo M, Liu Z, et al. Pancreatic cancer: BRCA mutation and personalized treatment. Expert Rev Anticancer Ther. 2015;15(10):1223–1231. doi: 10.1586/14737140.2015.1086271. [PubMed] [Cross Ref]81.
Moiseyenko VM, Volkov NM, Suspistin EN, Yanus GA, Iyevleva AG, Kuligina ES, et al. Evidence for predictive role of BRCA1 and bTUBIII in gastric cancer. Med Oncol. 2013;30(2):545. doi: 10.1007/s12032-013-0545-4. [PubMed] [Cross Ref]82.
De Summa S, Pinto R, Sambiasi D, Petriella D, Paradiso V, Paradiso A, et al. BRCAness: a deeper insight into basal-like breast tumors. Ann Oncol. 2013;24(Suppl 8):viii13–viii21. doi: 10.1093/annonc/mdt306. [PubMed] [Cross Ref]83.
Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16(2):110–120. doi: 10.1038/nrc.2015.21. [PubMed] [Cross Ref]84.
Hennessy BT, Timms KM, Carey MS, Gutin A, Meyer LA, Flake DD, 2nd, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28(22):3570–3576. doi: 10.1200/JCO.2009.27.2997. [PMC free article] [PubMed] [Cross Ref]85.
Stefansson OA, Villanueva A, Vidal A, Martí L, Esteller M. BRCA1 epigenetic inactivation predicts sensitivity to platinum-based chemotherapy in breast and ovarian cancer. Epigenetics. 2012;7(11):1225–1229. doi: 10.4161/epi.22561. [PMC free article] [PubMed] [Cross Ref]86.
Stecklein SR, Sharma P. Tumor homologous recombination deficiency assays: another step closer to clinical application? Breast Cancer Res. 2014;16(4):409. doi: 10.1186/s13058-014-0409-7. [PMC free article] [PubMed] [Cross Ref]87.
Timms KM, Abkevich V, Hughes E, Neff C, Reid J, Morris B, et al. Association of BRCA1/2 defects with genomic scores predictive of DNA damage repair deficiency among breast cancer subtypes. Breast Cancer Res. 2014;16(6):475. doi: 10.1186/s13058-014-0475-x. [PMC free article] [PubMed] [Cross Ref]88.
Watkins JA, Irshad S, Grigoriadis A, Tutt AN. Genomic scars as biomarkers of homologous recombination deficiency and drug response in breast and ovarian cancers. Breast Cancer Res. 2014;16(3):211. doi: 10.1186/bcr3670. [PMC free article] [PubMed] [Cross Ref]89.
De Picciotto N, Cacheux W, Roth A, Chappuis PO, Labidi-Galy SI. Ovarian cancer: Status of homologous recombination pathway as a predictor of drug response. Crit Rev Oncol Hematol. 2016;101:50–59. doi: 10.1016/j.critrevonc.2016.02.014. [PubMed] [Cross Ref]90.
Lips EH, Laddach N, Savola SP, Vollebergh MA, Oonk AM, Imholz AL, et al. Quantitative copy number analysis by Multiplex Ligation-dependent Probe Amplification (MLPA) of BRCA1-associated breast cancer regions identifies BRCAness. Breast Cancer Res. 2011;13(5):R107. doi: 10.1186/bcr3049. [PMC free article] [PubMed] [Cross Ref]91.
Vollebergh MA, Lips EH, Nederlof PM, Wessels LF, Wesseling J, Vd Vijver MJ, et al. Genomic patterns resembling BRCA1- and BRCA2-mutated breast cancers predict benefit of intensified carboplatin-based chemotherapy. Breast Cancer Res. 2014;16(3):R47. doi: 10.1186/bcr3655. [PMC free article] [PubMed] [Cross Ref]92.
Abkevich V, Timms KM, Hennessy BT, Potter J, Carey MS, Meyer LA, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107(10):1776–1782. doi: 10.1038/bjc.2012.451. [PMC free article] [PubMed] [Cross Ref]93.
Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li Q, Tian R, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2012;2(4):366–375. doi: 10.1158/2159-8290.CD-11-0206. [PMC free article] [PubMed] [Cross Ref]94.
Popova T, Manié E, Rieunier G, Caux-Moncoutier V, Tirapo C, Dubois T, et al. Ploidy and large-scale genomic instability consistently identify basal-like breast carcinomas with BRCA1/2 inactivation. Cancer Res. 2012;72(21):5454–5462. doi: 10.1158/0008-5472.CAN-12-1470. [PubMed] [Cross Ref]95.
Konstantinopoulos PA, Spentzos D, Karlan BY, Taniguchi T, Fountzilas E, Francoeur N, et al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol. 2010;28(22):3555–3561. doi: 10.1200/JCO.2009.27.5719. [PMC free article] [PubMed] [Cross Ref]96.
Severson TM, Peeters J, Majewski I, Michaut M, Bosma A, Schouten PC, et al. BRCA1-like signature in triple negative breast cancer: Molecular and clinical characterization reveals subgroups with therapeutic potential. Mol Oncol. 2015;9(8):1528–1538. doi: 10.1016/j.molonc.2015.04.011. [PubMed] [Cross Ref]97.
Shah MM, Dobbin ZC, Nowsheen S, Wielgos M, Katre AA, Alvarez RD, et al. An ex vivo assay of XRT-induced Rad51 foci formation predicts response to PARP-inhibition in ovarian cancer. Gynecol Oncol. 2014;134(2):331–337. doi: 10.1016/j.ygyno.2014.05.009. [PMC free article] [PubMed] [Cross Ref]98.
Schouten PC, Marmé F, Aulmann S, Sinn HP, van Essen HF, Ylstra B, et al. Breast cancers with a BRCA1-like DNA copy number profile recur less often than expected after high-dose alkylating chemotherapy. Clin Cancer Res. 2015;21(4):763–770. doi: 10.1158/1078-0432.CCR-14-1894. [PubMed] [Cross Ref]99.
Telli ML, Timms KM, Reid JE, Hennessy B, Mills GB, Jensen KC, et al Homologous Recombination Deficiency (HRD) Score Predicts Response to Platinum-Containing Neoadjuvant Chemotherapy in Patients with Triple Negative Breast Cancer. Clin Cancer Res. 2016 (in press). [PubMed]100.
Akashi-Tanaka S, Watanabe C, Takamaru T, Kuwayama T, Ikeda M, Ohyama H, et al. BRCAness predicts resistance to taxane-containing regimens in triple negative breast cancer during neoadjuvant chemotherapy. Clin Breast Cancer. 2015;15(1):80–85. doi: 10.1016/j.clbc.2014.08.003. [PubMed] [Cross Ref]101.
Hill SJ, Clark AP, Silver DP, Livingston DM. BRCA1 pathway function in basal-like breast cancer cells. Mol Cell Biol. 2014;34(20):3828–3842. doi: 10.1128/MCB.01646-13. [PMC free article] [PubMed] [Cross Ref]102.
Sakai W, Swisher EM, Karlan BY, Agarwal MK, Higgins J, Friedman C, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451(7182):1116–1120. doi: 10.1038/nature06633. [PMC free article] [PubMed] [Cross Ref]103.
Edwards SL, Brough R, Lord CJ, Natrajan R, Vatcheva R, Levine DA, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451(7182):1111–1115. doi: 10.1038/nature06548. [PubMed] [Cross Ref]104.
Swisher EM, Sakai W, Karlan BY, Wurz K, Urban N, Taniguchi T. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68(8):2581–2586. doi: 10.1158/0008-5472.CAN-08-0088. [PMC free article] [PubMed] [Cross Ref]105.
Sakai W, Swisher EM, Jacquemont C, Chandramohan KV, Couch FJ, Langdon SP, et al. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 2009;69(16):6381–6386. doi: 10.1158/0008-5472.CAN-09-1178. [PMC free article] [PubMed] [Cross Ref]106.
Norquist B, Wurz KA, Pennil CC, Garcia R, Gross J, Sakai W, et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol. 2011;29(22):3008–3015. doi: 10.1200/JCO.2010.34.2980. [PMC free article] [PubMed] [Cross Ref]107.
Barber LJ, Sandhu S, Chen L, Campbell J, Kozarewa I, Fenwick K, et al. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229(3):422–429. doi: 10.1002/path.4140. [PubMed] [Cross Ref]108.
Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–494. doi: 10.1038/nature14410. [PubMed] [Cross Ref]109.
Martins FC, De S, Almendro V, Gönen M, Park SY, Blum JL, et al. Evolutionary pathways in BRCA1-associated breast tumors. Cancer Discov. 2012;2(6):503–511. doi: 10.1158/2159-8290.CD-11-0325. [PMC free article] [PubMed] [Cross Ref]110.
Bardia A, Baselga J. Neoadjuvant therapy as a platform for drug development and approval in breast cancer. Clin Cancer Res. 2013;19(23):6360–6370. doi: 10.1158/1078-0432.CCR-13-0916. [PubMed] [Cross Ref]111.
Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy vs. primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–257. doi: 10.1016/S0140-6736(14)62223-6. [PubMed] [Cross Ref]112.
Sun CK, Zhang F, Xiang T, Chen Q, Pandita TK, Huang Y, et al. Phosphorylation of ribosomal protein S6 confers PARP inhibitor resistance in BRCA1-deficient cancers. Oncotarget. 2014;5(10):3375–3385. doi: 10.18632/oncotarget.1952. [PMC free article] [PubMed] [Cross Ref]113.
Henneman L, van Miltenburg MH, Michalak EM, Braumuller TM, Jaspers JE, Drenth AP, et al. Selective resistance to the PARP inhibitor olaparib in a mouse model for BRCA1-deficient metaplastic breast cancer. Proc Natl Acad Sci U S A. 2015;112(27):8409–8414. doi: 10.1073/pnas.1500223112. [PMC free article] [PubMed] [Cross Ref]114.
Guillemette S, Serra RW, Peng M, Hayes JA, Konstantinopoulos PA, Green MR, et al. Resistance to therapy in BRCA2 mutant cells due to loss of the nucleosome remodeling factor CHD4. Genes Dev. 2015;29(5):489–494. doi: 10.1101/gad.256214.114. [PMC free article] [PubMed] [Cross Ref]115.
Schouten PC, Vollebergh MA, Opdam M, Jonkers M, Loden M, Wesseling J, et al. High XIST and Low 53BP1 Expression Predict Poor Outcome after High-Dose Alkylating Chemotherapy in Patients with a BRCA1-like Breast Cancer. Mol Cancer Ther. 2016;15(1):190–198. doi: 10.1158/1535-7163.MCT-15-0470. [PubMed] [Cross Ref]116.
Lake DE, Hudis CA. High-dose chemotherapy in breast cancer. Drugs. 2004;64(17):1851–1860. doi: 10.2165/00003495-200464170-00001. [PubMed] [Cross Ref]117.
Pedrazzoli P, Ledermann JA, Lotz JP, Leyvraz S, Aglietta M, Rosti G, European Group for Blood and Marrow Transplantation (EBMT) Solid Tumors Working Party et al. High dose chemotherapy with autologous hematopoietic stem cell support for solid tumors other than breast cancer in adults. Ann Oncol. 2006;17(10):1479–1488. doi: 10.1093/annonc/mdl044. [PubMed] [Cross Ref]118.
Banna GL, Simonelli M, Santoro A. High-dose chemotherapy followed by autologous hematopoietic stem-cell transplantation for the treatment of solid tumors in adults: a critical review. Curr Stem Cell Res Ther. 2007;2(1):65–82. doi: 10.2174/157488807779316964. [PubMed] [Cross Ref]119.
Vollebergh MA, Nederlof PM, Wessels LF, Schmidt MK, Joosse SA, van Beers E, et al. Predicting response to alkylating chemotherapy in breast cancer patients using array comparative genomic hybridization. Cancer Res. 2009;69(Suppl 1):abstract 6050. doi: 10.1158/0008-5472.SABCS-6050. [Cross Ref]120.
Vollebergh MA, Lips EH, Nederlof PM, Wessels LF, Schmidt MK, van Beers EH, et al. An aCGH classifier derived from BRCA1-mutated breast cancer and benefit of high-dose platinum-based chemotherapy in HER2-negative breast cancer patients. Ann Oncol. 2011;22:1561–1570. doi: 10.1093/annonc/mdq624. [PMC free article] [PubMed] [Cross Ref]121.
Huang F, Kushner YB, Langleben A, Foulkes WD. Medscape: Eleven years disease-free: role of chemotherapy in metastatic BRCA2-related breast cancer. Nat Rev Clin Oncol. 2009;6:488–492. doi: 10.1038/nrclinonc.2009.90. [PubMed] [Cross Ref]122.
Steenbruggen TG, Linn SC, Rodenhuis S, Sonke GS. Ongoing Remission Nineteen Years after High-dose Chemotherapy for Oligometastatic Breast Cancer; What Can We Learn from this Patient? Cureus. 2015;7(12):e433. [PMC free article] [PubMed]123.
Konishi H, Mohseni M, Tamaki A, Garay JP, Croessmann S, Karnan S, et al. Mutation of a single allele of the cancer susceptibility gene BRCA1 leads to genomic instability in human breast epithelial cells. Proc Natl Acad Sci U S A. 2011;108(43):17773–17778. doi: 10.1073/pnas.1110969108. [PMC free article] [PubMed] [Cross Ref]124.
Drooger JC, Heemskerk-Gerritsen BA, Smallenbroek N, Epskamp C, Seynaeve CM, Jager A. Toxicity of (neo)adjuvant chemotherapy for BRCA1- and BRCA2-associated breast cancer. Breast Cancer Res Treat. 2016;156(3):557–566. doi: 10.1007/s10549-016-3777-0. [PMC free article] [PubMed] [Cross Ref]125.
Moon DH, Lee JM, Noonan AM, Annunziata CM, Minasian L, Houston N, et al. Deleterious BRCA1/2 mutation is an independent risk factor for carboplatin hypersensitivity reactions. Br J Cancer. 2013;109(4):1072–1078. doi: 10.1038/bjc.2013.389. [PMC free article] [PubMed] [Cross Ref]126.
Bernier J, Poortmans P. Clinical relevance of normal and tumour cell radiosensitivity in BRCA1/BRCA2 mutation carriers: a review. Breast. 2015;24(2):100–106. doi: 10.1016/j.breast.2014.12.003. [PubMed] [Cross Ref]127.
Drooger JC, Hooning MJ, Seynaeve CM, Baaijens MH, Obdeijn IM, Sleijfer S, et al. Diagnostic and therapeutic ionizing radiation and the risk of a first and second primary breast cancer, with special attention for BRCA1 and BRCA2 mutation carriers: a critical review of the literature. Cancer Treat Rev. 2015;41(2):187–196. doi: 10.1016/j.ctrv.2014.12.002. [PubMed] [Cross Ref]128.
Suspitsin EN, Yanus GA, Sokolenko AP, Yatsuk OS, Zaitseva OA, Bessonov AA, et al. Development of breast tumors in CHEK2, NBN/NBS1 and BLM mutation carriers does not commonly involve somatic inactivation of the wild-type allele. Med Oncol. 2014;31(2):828. doi: 10.1007/s12032-013-0828-9. [PubMed] [Cross Ref]129.
Chrisanthar R, Knappskog S, Løkkevik E, Anker G, Østenstad B, Lundgren S, et al. CHEK2 mutations affecting kinase activity together with mutations in TP53 indicate a functional pathway associated with resistance to epirubicin in primary breast cancer. PLoS One. 2008;3(8):e3062. doi: 10.1371/journal.pone.0003062. [PMC free article] [PubMed] [Cross Ref]130.
Kriege M, Jager A, Hollestelle A, Berns EM, Blom J, Meijer-van Gelder ME, et al. Sensitivity to systemic therapy for metastatic breast cancer in CHEK2 1100delC mutation carriers. J Cancer Res Clin Oncol. 2015;141(10):1879–1887. doi: 10.1007/s00432-015-1981-7. [PMC free article] [PubMed] [Cross Ref]131.
Sokolenko AP, Suspitsin EN, Kuligina ES, Bizin IV, Frishman D, Imyanitov EN. Identification of novel hereditary cancer genes by whole exome sequencing. Cancer Lett. 2015;369(2):274–288. doi: 10.1016/j.canlet.2015.09.014. [PubMed] [Cross Ref]132.
De Smedt L, Lemahieu J, Palmans S, Govaere O, Tousseyn T, Van Cutsem E, et al. Microsatellite instable vs stable colon carcinomas: analysis of tumour heterogeneity, inflammation and angiogenesis. Br J Cancer. 2015;113(3):500–509. doi: 10.1038/bjc.2015.213. [PMC free article] [PubMed] [Cross Ref]133.
Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol. 2015;16(7):30. doi: 10.1007/s11864-015-0348-2. [PMC free article] [PubMed] [Cross Ref]134.
Stadler ZK. Diagnosis and management of DNA mismatch repair-deficient colorectal cancer. Hematol Oncol Clin North Am. 2015;29(1):29–41. doi: 10.1016/j.hoc.2014.09.008. [PubMed] [Cross Ref]135.
Perucho M. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248-57. Cancer Res. 1999;59:249–56. [PubMed]136.
Devaud N, Gallinger S. Chemotherapy of MMR-deficient colorectal cancer. Fam Cancer. 2013;12(2):301–306. doi: 10.1007/s10689-013-9633-z. [PubMed] [Cross Ref]137.
Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5(1):43–51. doi: 10.1158/2159-8290.CD-14-0863. [PMC free article] [PubMed] [Cross Ref]138.
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [PMC free article] [PubMed] [Cross Ref]139.
Laghi L, Malesci A. Microsatellite instability and therapeutic consequences in colorectal cancer. Dig Dis. 2012;30(3):304–309. doi: 10.1159/000337003. [PubMed] [Cross Ref]140.
Guillotin D, Martin SA. Exploiting DNA mismatch repair deficiency as a therapeutic strategy. Exp Cell Res. 2014;329(1):110–115. doi: 10.1016/j.yexcr.2014.07.004. [PubMed] [Cross Ref]141.
Samadder NJ, Neklason DW, Boucher KM, Byrne KR, Kanth P, Samowitz W, et al. Effect of Sulindac and Erlotinib vs Placebo on Duodenal Neoplasia in Familial Adenomatous Polyposis: A Randomized Clinical Trial. JAMA. 2016;315(12):1266–1275. doi: 10.1001/jama.2016.2522. [PMC free article] [PubMed] [Cross Ref]142.
Wells SA, Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010;28(5):767–772. doi: 10.1200/JCO.2009.23.6604. [PMC free article] [PubMed] [Cross Ref]143.
Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31(29):3639–3646. doi: 10.1200/JCO.2012.48.4659. [PMC free article] [PubMed] [Cross Ref]144.
Fox E, Widemann BC, Chuk MK, Marcus L, Aikin A, Whitcomb PO, et al. Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res. 2013;19(15):4239–4248. doi: 10.1158/1078-0432.CCR-13-0071. [PMC free article] [PubMed] [Cross Ref]145.
Wells SA, Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012;30(2):134–141. doi: 10.1200/JCO.2011.35.5040. [PMC free article] [PubMed] [Cross Ref]146.
Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363(19):1801–1811. doi: 10.1056/NEJMoa1001671. [PubMed] [Cross Ref]147.
Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381(9869):817–824. doi: 10.1016/S0140-6736(12)61767-X. [PubMed] [Cross Ref]148.
Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2-year open-label extension of the randomised EXIST-1 study. Lancet Oncol. 2014;15(13):1513–1520. doi: 10.1016/S1470-2045(14)70489-9. [PubMed] [Cross Ref]149.
Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366(23):2180–2188. doi: 10.1056/NEJMoa1113538. [PMC free article] [PubMed] [Cross Ref]150.
Ally MS, Tang JY, Joseph T, Thompson B, Lindgren J, Raphael MA, et al. The use of vismodegib to shrink keratocystic odontogenic tumors in patients with basal cell nevus syndrome. JAMA Dermatol. 2014;150(5):542–545. doi: 10.1001/jamadermatol.2013.7444. [PMC free article] [PubMed] [Cross Ref]151.
Tang JY, Aszterbaum M, Athar M, Barsanti F, Cappola C, Estevez N, et al. Basal cell carcinoma chemoprevention with nonsteroidal anti-inflammatory drugs in genetically predisposed PTCH1+/- humans and mice. Cancer Prev Res (Phila) 2010;3(1):25–34. doi: 10.1158/1940-6207.CAPR-09-0200. [PMC free article] [PubMed] [Cross Ref]152.
Kim J, Tang JY, Gong R, Kim J, Lee JJ, Clemons KV, et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17(4):388–399. doi: 10.1016/j.ccr.2010.02.027. [PMC free article] [PubMed] [Cross Ref]153.
Chen B, Trang V, Lee A, Williams NS, Wilson AN, Epstein EH Jr, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the Hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016 (in press). [PMC free article] [PubMed]154.
Kim DJ, Kim J, Spaunhurst K, Montoya J, Khodosh R, Chandra K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32(8):745–751. doi: 10.1200/JCO.2013.49.9525. [PubMed] [Cross Ref]155.
Ally MS, Ransohoff K, Sarin K, Atwood SX, Rezaee M, Bailey-Healy I, et al. Effects of Combined Treatment With Arsenic Trioxide and Itraconazole in Patients With Refractory Metastatic Basal Cell Carcinoma. JAMA Dermatol. 2016;152(4):452–456. doi: 10.1001/jamadermatol.2015.5473. [PMC free article] [PubMed] [Cross Ref]156.
Coombes RC. Drug testing in the patient: toward personalized cancer treatment. Sci Transl Med. 2015;7(284):284ps10. doi: 10.1126/scitranslmed.aab1214. [PubMed] [Cross Ref]157.
Menden MP, Iorio F, Garnett M, McDermott U, Benes CH, Ballester PJ, et al. Machine learning prediction of cancer cell sensitivity to drugs based on genomic and chemical properties. PLoS One. 2013;8(4):e61318. doi: 10.1371/journal.pone.0061318. [PMC free article] [PubMed] [Cross Ref]
Articles from Hereditary Cancer in Clinical Practice are provided here courtesy of BioMed Central
BRCA, BRCA-like, Mini-CTC, mini stamceltransplantatie, borstkanker, olaparib, Doxorubicin, Endoxan, CYCLOPHOSPHAMIDE, carboplatin, personalised medicin, Minster SChippers, basisverzekering, placebo
Gerelateerde artikelen




 1,2,3,4
1,2,3,4
Plaats een reactie ...
Reageer op "Mini stamceltransplantatie (mini-CTC) plus chemo gevolgd door olaparib voor borstkanker stadium III met BRCA-like status komt in basisverzekering"