Raadpleeg ook de literatuurlijst niet-toxische ondersteuning van arts-bioloog drs. Engelbert Valstar bij borstkanker.
2 september 2025: zie ook dit artikel: https://kanker-actueel.nl/olaparib-48-uur-gegeven-na-chemotherapie-vooraf-aan-operatie-bij-borstkankerpatienten-met-brca-1-of-2-status-zorgt-voor-ziektevrije-overleving-van-alle-39-deelnemende-borstkankerpatienten-op-3-jaars-meting-vanaf-operatiedatum.html
13 augustus 2021: zie ook dit artikel: https://kanker-actueel.nl/olaparib-een-parpremmer-gegeven-aan-borstkankerpatienten-met-brca-1-en-brca-2-na-operatie-en-chemotherapie-verbetert-ziektevrije-overleving-met-9-procent.html
Zie ook dit artikel:
Zie ook in gerelateerde artikelen
6 mei 2023: Uit nieuwe gegevens van de OlympiAD en de EMBRACA studies blijkt dat Olaparib toch betere resultaten laat zien in vergelijking met beste keuze van een oncoloog. Uit de laatste follow-up:
Belangrijkste punten uit de studie: :
- De mediane totale overleving (OS) was respectievelijk 19,3 en 17,1 maanden voor olaparib versus chemotherapie naar keuze van de arts.
- In totaal ontving 8,8% van de patiënten ten minste 3 jaar studiebehandeling in de olaparib groep versus geen patiënten in de TPC groep.
- In de eerste lijn was de mediane OS numeriek langer voor olaparib versus TPC (respectievelijk 22,6 versus 14,7 maanden).
- In de eerste lijn was het OS-percentage na 3 jaar 40,8% voor olaparib versus 12,8% voor TPC.
Deze gegevens bevestigen eerdere bevindingen en suggereren een mogelijk OS-voordeel voor olaparib bij eerstelijns gemetastaseerde borstkanker. Zie verder abstract onderaaan artikel en verder hier in dit artikel:
26 augustus 2018: Bron: Bron ESMO open
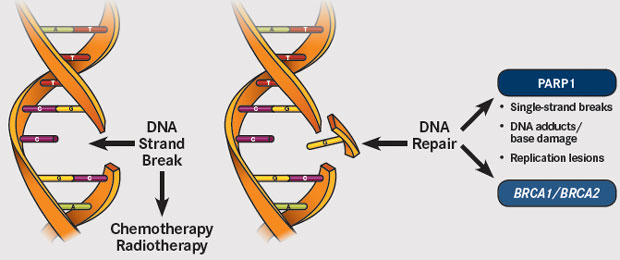
Voor borstkankerpatiënten met een erfelijke vorm van kanker met BRCA-1 en BRCA-2 mutaties en HER-2 negatieve expressie zijn PARP remmers, zoals olaparib en rucaparib en niraparib enz., de aangewezen behandeling als er sprake is van een recidief en uitzaaiingen na eerste- en tweedelijns behandelingen.
Echter nu blijkt uit een meta analyse en reviewstudie van twee gerandomiseerde studies met totaal 733 patiënten, dat weliswaar de progressievrije ziekte met enkele maanden werd verlengd en de kwaliteit van leven wel wat beter was voor de PARP groep. (In de subgroep analyse gerelateerd aan de hormoon receptor status, single-agent PARPi was geassocieerd met verbeterde progressievrije ziekte (PFS) met alleen in de hormoonnegatieve receptorgroep een statistisch significant verschil. (HR 0.51 (95% CI 0.37 to 0.71, p<0.001)) (I2=31.5%, p=0.227; figure 3A en niet in de hormoon receptor positieve groep (HR 0.62 (95% CI 0.36 to 1.07, p=0.085)) (I2=72.2%, p=0.058; figure 3B.)
Maar de uiteindelijke overall overleving verschilde niet in vergelijking met chemotherapie. (In de OlympiAD en de EMBRACA studies, respectievelijk, mediane OS was 19.3 maanden en 22.3 maanden in de PARPi groepen versus 19.6 maanden en 19.5 maanden in de chemotherapie groep).

Dit is de kernboodschap van deze reviewstudie:
-
This meta-analysis of two randomized controlled trials including 733 patients was designed to assess the safety, activity, and efficacy of single-agent PARP inhibitors compared with monochemotherapy in patients with BRCA-mutated HER2-negative metastatic breast cancer. Significant improvements in progression-free survival and objective response rates were observed with single-agent PARP inhibitors compared with chemotherapy. Patients treated with PARP inhibitors also reported a significant delay in time to quality-of-life deterioration. PARP inhibitor use led to a significantly increased risk of headache and anemia, and a decreased risk of palmar–plantar erythrodysesthesia syndrome and neutropenia compared with monochemotherapy.
-
These findings demonstrate the tolerability and efficacy of single-agent PARP inhibitors in this patient population.
 Source: Onclive.com
Source: Onclive.com
Klik op de titel van de studie om het volledige studierapport in te zien dat gratis is met veel verduidelijkende grafieken:
Abstract
Single-agent poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi) have been approved as the first targeted therapy available for patients with BRCA-mutated HER2-negative metastatic breast cancer. This meta-analysis aimed to better evaluate activity, efficacy and safety of single-agent PARPi in this population. A systematic search of Medline, Embase and conference proceedings up to 31 January 2018 was conducted to identify randomised controlled trials (RCTs) investigating single-agent PARPi versus monochemotherapy in patients with BRCA-mutated HER2-negative metastatic breast cancer. Using the random-effect model, we calculated summary risk estimates (pooled HR and OR with 95% CI) for progression-free survival (PFS), overall survival (OS), objective response rate (ORR), any grade and grade 3–4 adverse events (AEs), treatment discontinuation rate and time to deterioration in quality of life (QoL). Two RCTs (n=733) were included. As compared with monochemotherapy, single-agent PARPi significantly improved PFS (HR 0.56(95% CI 0.45 to 0.70)) and ORR (OR 4.15 (95% CI 2.82 to 6.10)), with no difference in OS (HR 0.82 (95% CI 0.64 to 1.05)). Single-agent PARPi significantly increased risk of anaemia and any grade headache, but reduced risk of neutropenia and any grade palmar-plantar erythrodysesthesia syndrome as compared with monochemotherapy. No significant differences in other AEs and treatment discontinuation rate were observed. Patients treated with PARPi experienced a significant delayed time to QoL deterioration (HR 0.40 (95% CI 0.29 to 0.54)). Single-agent PARPi showed to be an effective, well tolerated and useful treatment in maintaining QoL of patients with BRCA-mutated HER2-negative metastatic breast cancer.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Acknowledgments
ML thanks the European Society for Medical Oncology (ESMO) for support in the form of a Translational Research Fellowship at the Institut Jules Bordet (Brussels, Belgium).
References
Footnotes
-
Contributors All the authors make substantial contributions to conception and design, and/or acquisition of data, and/or analysis and interpretation of data, giving final approval of the version to be submitted.
-
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
-
Competing interests LDM received personal fees from Novartis Pharma AG, Roche-Genentech, Ipsen, Astrazeneca, Takeda, Eli Lilly outside the submitted work. EdA received honoraria from Roche-Genentech, research grant from Roche-Genentech (to the institution) and travel grants from Roche-Genentech and GlaxoSmithKline outside the submitted work. ML served as a consultant for Teva outside the submitted work.
-
Patient consent Not required.
-
Provenance and peer review Not commissioned; internally peer reviewed.
Request Permissions
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Copyright information:
In first-line, the 3-year OS rate was 40.8% for olaparib versus 12.8% for treatment of physician's choice (TPC).
OlympiAD extended follow-up for overall survival and safety: Olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer
Highlights
- •
Median overall survival (OS) was 19.3 and 17.1 months for olaparib versus chemotherapy treatment of physician's choice (TPC), respectively.
- •
Overall, 8.8% of patients received at least 3 years of study treatment in the olaparib arm versus no patients in the TPC arm.
- •
In first-line, median OS was numerically longer for olaparib versus TPC (22.6 versus 14.7 months, respectively).
- •
In first-line, the 3-year OS rate was 40.8% for olaparib versus 12.8% for TPC.
- •
These data confirm previous findings and suggest a possible OS benefit for olaparib in the first-line metastatic breast cancer setting.
Abstract
Background
Patients and methods
Results
Conclusions
References
-
Prevalence and predictors of BRCA1 and BRCA2 mutations in a population-based study of breast cancer in white and black American women ages 35 to 64 years.Cancer Res. 2006; 66: 8297-8308
-
Performance of prediction models for BRCA mutation carriage in three racial/ethnic groups: findings from the Northern California Breast Cancer Family Registry.Cancer Epidemiol Biomark Prev. 2009; 18: 1084-1091
-
Targeted sequencing of BRCA1 and BRCA2 across a large unselected breast cancer cohort suggests that one-third of mutations are somatic.Ann Oncol. 2016; 27: 1532-1538
-
Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer.J Clin Oncol. 2015; 33: 304-311
-
Unique features of young age breast cancer and its management.J Breast Cancer. 2014; 17: 301-307
-
Low prevalence of HER2 positivity amongst BRCA1 and BRCA2 mutation carriers and in primary BRCA screens.Breast Cancer Res Treat. 2016; 155: 597-601
-
Olaparib for metastatic breast cancer in patients with a germline BRCA mutation.N Engl J Med. 2017; 377: 523-533
-
Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial.Eur J Cancer. 2019; 120: 20-30
-
OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer.Ann Oncol. 2019; 30: 558-566
-
Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy.Br J Cancer. 2018; 119: 1075-1085
-
Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial.Ann Oncol. 2020; 31: 1526-1535
Article info
Publication history
Identification
Copyright
ScienceDirect
Access this article on ScienceDirectRelated Articles
- Clinical effectiveness of olaparib monotherapy in germline BRCA-mutated, HER2-negative metastatic breast cancer in a real-world setting: phase IIIb LUCY interim analysis
European Journal of CancerJune 2, 2021Open Access
- Patient-reported outcomes in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer receiving olaparib versus chemotherapy in the OlympiAD trial
European Journal of CancerAugust 22, 2019Open Access
- Overall survival with palbociclib plus endocrine therapy versus capecitabine in postmenopausal patients with hormone receptor-positive, HER2-negative metastatic breast cancer in the PEARL study
European Journal of CancerApril 14, 2022Open Access
- Q-TWiST analysis of pembrolizumab combined with chemotherapy as first-line treatment of metastatic triple-negative breast cancer that expresses PD-L1
European Journal of CancerOctober 29, 2022
- Survival outcomes after neoadjuvant letrozole and palbociclib versus third generation chemotherapy for patients with high-risk oestrogen receptor-positive HER2-negative breast cancer
European Journal of CancerMarch 22, 2022
Gerelateerde artikelen
- Olaparib 48 uur gegeven na chemotherapie vooraf aan operatie bij borstkankerpatienten met BRCA 1 of 2 status zorgt voor ziektevrije overleving van alle 39 deelnemende borstkankerpatienten op 3 jaars meting vanaf operatiedatum
- PARP-remmers geven bij patiënten met uitgezaaide borstkanker met gPALB2 en sBRCA mutaties maar in slechte lichamelijke gesteldheid alsnog goede resultaten
- BRIP1 mutatie geeft uitstekende respons op olaparib bij uitgezaaide HR-positieve, HER2-negatieve borstkanker blijkt uit casestudie copy 2
- Olaparib, een parpremmer, gegeven aan borstkankerpatienten met BRCA 1 en BRCA 2 na operatie en chemotherapie verbetert ziektevrije overleving met 9 procent
- Olaparib in combinatie met de PD-L1-remmer, durvalumab, geeft hoopvolle resultaten bij patiënten met erfelijke BRCA1-gemuteerde of BRCA2-gemuteerde uitgezaaide borstkanker.
- Mini stamceltransplantatie (mini-CTC) plus chemo gevolgd door olaparib voor borstkanker stadium III met BRCA-like status komt in basisverzekering
- neratinib plus capecitabine (Xeloda) blijkt alsnog therapeutisch effect te geven bij recidief van HER2-positieve borstkanker met uitzaaiingen in de hersenen
- Reguliere behandelingen, medicijnen en middelen bij borstkanker: een overzicht



Plaats een reactie ...
Reageer op "PARP remmers als monotherapie voor BRCA-1 en BRCA 2 pos. plus HER2-neg. uitgezaaide borstkanker geeft wel langere progressievrije ziekte maar geen verschil in overall overleving in vergelijking met chemotherapie"