Helpt u ons aan 500 donateurs?
22 november 2018: Lees ook dit artikel:
25 januari 2018: Bron: Medicine (Baltimore). 2017 Nov; 96(46): e8731: Published online 2017 Nov 17.
Jaren geleden maakte ik een reportage over de maretak in Duitsland. (of zie in gerelateerde artikelen). Hier een bijzonder ervaringsverhaal en uitstekend gedocumenteerde case studie over het effect van maretak injecties bij een kankerpatiënt.
Een nu 66 jarige vrouw die in 2012 werd gediagnosteerd met een recidief van eerder chirurgisch verwijderde melanoom maar nu in de lymfklieren uitgezaaide melanoom weigerde chemo en koos voor immuuntherapie met maretakinjecties met hoge dosis Viscum Album. En met een uitstekend resultaat want zij kwam binnen ca. 2 jaar maretakinjecties in een complete remissie en is dat nu al ruim 5 jaar. Na ca. 1,5 jaar vanaf de start van de maretakinjecties waren alle zichtbare tumoren verdwenen. Inmiddels is de vrouw nu dus al 5 jaar verder en blijkt nog steeds kankervrij. En voelt zich uitstekend.
Zie hier Petscan beeld voor en na de maretakinjecties. (tekst gaat verder onder scanbeeld.
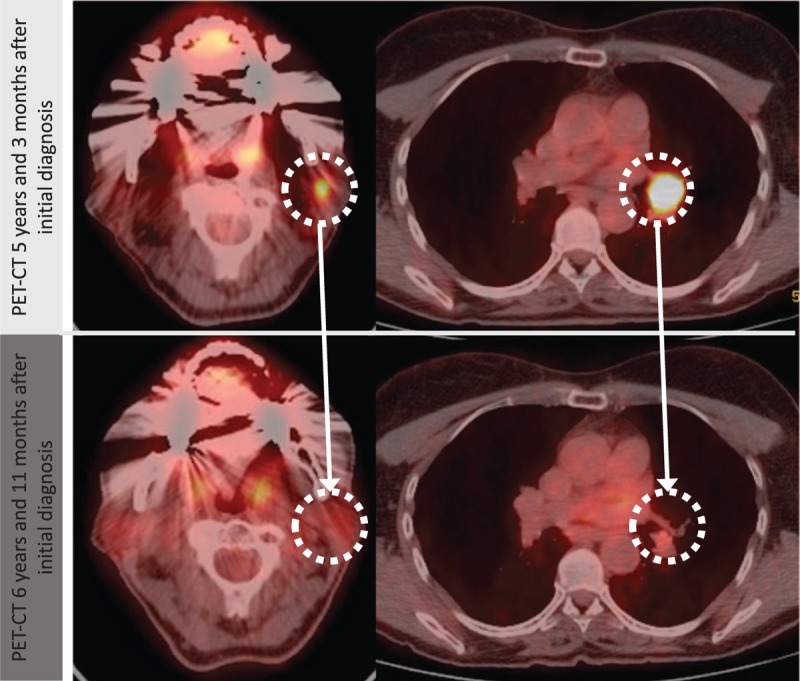
De vrouw had voor het melanoom bij haar werd geconstateerd dus al eerder een melanoom gehad en deze was chirurgisch verwijderd. 4 jaar later (2012) kreeg ze een recidief met uitzaaiingen in de lymfklieren. Het melanoom was BRAF negatief waardoor ze niet in aanmerking kwam voor immuuntherapie met een anti-PD medicijn. Ook had ze eerder in haar leven veel problemen met haar gezondheid gehad. De vrouw is onderwijzeres en koos heel bewust voor de maretakinjecties en daarnaast een gezonde leefstijl en wist heel goed wat voor risico's ze nam. Maar zij deed dat bewust.
Aanvullend gebruikte de vrouw ook voedingssupplementen, met name sodium, calcium, potassium, en magnesium, en zij gebruikte ook visoliecapsules.
Wat ik mooi vind is dat in deze case studie zij ook haar persoonlijke keuze toelicht. Ik vertaal deze maar niet want ik wil geen fouten maken in de vertaling (anders vertaalt u zelf met google translation rechtsboven dit artikel).
4. Patient's view
Following the diagnosis of the metastases, I was told at the University Hospital that it would most likely be a month before further therapy was continued. During this time, I found out about intensive Mistletoe Therapy and the hospital offering Anthroposophic Medicine. I learnt that I could have confidence in myself and that I could contribute to my own self-healing process. I realized that my own commitment to becoming healthy was needed, and learned to be grateful for everything that was healthy in me. This commitment to my own health also involved the development of an awareness of my life, including uncovering all those things which were sabotaging me from the inside. Because of this, I was able to find and take my own path in therapy—even if there were doctors who wanted to convince me not to take this path. I was able to tolerate the Mistletoe Therapy, with its high fever and side effects, as well as the sometimes painful injections, because I felt safe and confident in the treatment.
Hier uit het studierapport een grafiek over de loop van de tijd die uitstekend weergeeft hoe haar proces verliep. Zij is altijd onder controle gebleven van haar behandelend arts.

Ook uit het studierapport een grafiek hoe de koorts bij haar verliep, want de hoge dosis kan dus soms hoge koorts veroorzaken:

Conclusie
Op basis van deze case studie en eerder gemelde individuele patiënten ervaringen lijkt een hoge dosis Viscum Album (VAE) een positief therapeutisch effect te hebben bij uitgezaaide melanomen ( MCM), vooral bij gebruik in hoge doseringen. Van ML en VT is bekend dat ze directe cytotoxische eigenschappen hebben en effect hebben op immuunwegen van het specifieke en niet-specifieke immuunsysteem en zouden op deze manier kunnen hebben bijgedragen aan de tumorcontrole in dit geval. Verder onderzoek naar de veiligheid en effectiviteit van hooggedoseerde en intraveneuze en intralesionale VAE, met name in MCM, is geboden
In het volledige studierapport: Complete remission and long-term survival of a patient with melanoma metastases treated with high-dose fever-inducing Viscum album extract wordt gedetailleerd beschreven hoe de vrouwm is behandeld en wat zij daarnaast nog meer nam aan voedingssupplementen. Hier het abstract met referentielijst.
Complete remission and long-term survival of a patient with melanoma metastases treated with high-dose fever-inducing Viscum album extract
Complete remission and long-term survival of a patient with melanoma metastases treated with high-dose fever-inducing Viscum album extract
Abstract
Introduction:
Metastatic malignant cutaneous melanoma (MCM)—a highly immunogenic cancer—typically has a poor prognosis. Viscum album extracts (VAEs) have strong immune-stimulating, apoptogenic, and cytotoxic effects.
Case presentation:
A 66-year-old MCM patient with newly diagnosed lymph node metastases opted for sole VAE treatment. VAEs were initially applied subcutaneously, and then later in exceptionally high, fever-inducing doses, both intravenously and intralesionally. The metastases shrunk over the following months, and after 2 years, all lesions had completely remitted (regional and hilar lymph nodes). The patient has been tumor free for 3.5 years at the time of publication (and for 5 years since initiation of intensified VAE treatment). Besides fever and flu-like symptoms, no side effects occurred.
Discussion:
We presume that VAE triggered an increased release of tumor-associated antigens, enhanced immunologic recognition, and increased immune response against the tumor tissue and induced tumor remission.
7. Conclusion
On the basis of this case and earlier reported cases, VAE seems to have a positive potential in MCM, especially when used in high dosages. ML and VT are known to have direct cytotoxic properties and stimulate immune pathways of the specific and unspecific immune system and in this way might have contributed to tumor control in this case. Further research on the safety and effectiveness of high-dose and intravenous and intralesional VAE, particularly in MCM, is warranted.
Acknowledgments
We thank the research department of the Klinik Arlesheim for providing the temperature data of the patient and for practical help, Dr. Helmut Kiene for revision of the manuscript, and the “Stiftung Integrative Medizin” for financial support. This case report was prepared following the CARE Guidelines.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, BRAF = rapidly growing fibrosarcoma type B gene, FDG = Fludeoxyglucose (18F), MCM = malignant cutaneous melanoma, ML = mistletoe lectin, PET-CT = positron emission tomography–computed tomography, UICC = Union internationale contre le cancer, VAE = Viscum album extracts, VT = viscotoxin.
Authorship: PGW, AH, and GSK contributed to the case report design. AH was the physician in charge and provided the patient's information. PGW and AH collected and provided the data. PGW was the principle author of the paper, had full access to all data, and is the guarantor. GSK supervised the case report and publication processes.
Funding/support: This case report was prepared following the CARE Guidelines.
Informed consent was received from the patient for the publication of the report and accompanying images. The patient read the submission version of the report and confirmed its content.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: IFAEMM has received restricted research grants, honoraria, and travel expenses from Weleda, Abnoba, and Helixor. None of them had any influence on the design, conduction, analysis, and publication of the study. AH declared no conflict of interest.
References
Gerelateerde artikelen
- Voorlichtingsfilm over de productie en het gebruik van de maretak (Viscum Album) bij kankerpatienten
- Maretak extract (Viscum Album) naast chemo-radiotherapie bij patienten met rectumkanker geeft betere resultaten op complete en gedeeltelijke remissies dan zonder maretak extract
- Maretakextracten, meestal Viscum Album gebruikt als aanvullende therapie voor kankerpatiënten, hebben een positieve invloed op de kwaliteit van leven blijkt uit reviewstudie van gerandomiseerde studies
- Maretakinjecties hoge dosis doen kanker volledig verdwijnen bij vrouw met recidief van Merkelcel carcinoom (75 jaar) en bij vrouw met borstkanker in beide borsten (50 jaar) zonder andere behandelingen. Beide vrouwen leven al jaren zonder kanker
- Immuuntherapie met maretak injecties met hoge dosis Viscum Album extract geeft volledige remissie en langjarige blijvende overleving van een patiënt met uitgezaaide melanoom.
- Maretakinjecties - Viscum album L. verdubbelt mediane overleving bij uitgezaaide, inoperabele alvleesklierkanker met betere kwaliteit van leven copy 1
- Maretak injecties geven een uitstekend resultaat bij borstkanker en gynaecologische vormen van kanker, blijkt uit grote overzichtstudie.
- Maretak injecties brengen Nederlandse vrouw met lymfklierkanker - non-Hodgkin in totale remissie na eerdere weigering van chemo. Artikel update 15 augustus 2010
- Maretak - Viscum Album (bv. Iscador, Abnoba): een veel gebruikt middel in een behandeling tegen kanker. Een overzicht van studies met kankerpatienten.
- Maretak injecties - Viscum Album L. (merknaam Iscador) naast reguliere behandelingen geeft significant betere resultaten op overlevingscijfers en ziekteproces en bijwerkingen bij melanoompatiënten met stadium II en III, zonder uitzaaiïngen op afstand
- Maretak injecties - Viscum Album L. (Iscador) naast reguliere behandelingen geeft significant betere resultaten op overlevingscijfers en ziekteproces en bijwerkingen bij borstkankerpatiënten.
- Maretak - Viscum Album L. (Isorel) gegeven vooraf en na operatie van kankerpatienten met spijsverteringskanker zoals darmkanker, alvleesklierkanker, slokdarmkanker en galwegenkanker geeft significant betere mediane overleving en kwaliteit van leven
- Maretakinjecties als immuuntherapie: Informatie over rol en effect van Maretak - Viscum Album L. (bv. merknamen Iscador of Isorel) in een behandeling of preventie van kanker



Plaats een reactie ...
Reageer op "Immuuntherapie met maretak injecties met hoge dosis Viscum Album extract geeft volledige remissie en langjarige blijvende overleving van een patiënt met uitgezaaide melanoom."