16 juli 2020: lees ook dit artikel:
24 augustus 2017: zie ook dit artikel:
25 december 2015: Bron: N Engl J Med. 2015 Nov 5;373(19):1803-13. doi: 10.1056/NEJMoa1510665. en FDA website approvals
Nivolumab (Obdivo) , een zogeheten anti PD medicijn (anti programmed death medicijn), en als zodanig ook als immuuntherapeutisch medicijn aangemerkt omdat deze medicijnen de apoptose (zelfdoding) van de kankercel stimuleren, is door de FDA - Food and Drug Administration goedgekeurd om als tweedelijns behandeling te gebruiken bij gevorderde nierkanker na progressie van de ziekte na behandelingen plus avastin - bevacizumab.
Klik hier voor de goedkeuringstekst van de FDA per 23 november 2015.
De goedkeuring van de FDA is verstrekt op basis van de resultaten uit een langjarige grote fase III studie bij totaal 821 patiënten met gevorderde zogeheten 'clear-cell renal-cell carcinoma, veruit de meest voorkomende vorm van nierkanker, waarbij nivolumab werd vergeleken met everolimus, een medicijn dat al eerder goede resultaten had laten zien in vergelijking met andere eerdere behandelingen voor nierkanker.
De overall overleving was 25 maanden voor nivolumab (en kan nog beter worden omdat einde studie-follow-up voor nivolumab nog niet is bereikt) tegenover 19,6 maanden voor Everolimus. Van de 410 patiënten bereikte 21,5 % een complete remissie of gedeeltelijke remissie (50% of meer minder tumoren en/of tumorgrootte) in de nivolumabgroep tegenover slechts 3,9% in de everolimusgroep (Afinitor)
Uit studieresults: Additionally, 21.5 percent of those treated with Opdivo experienced a complete or partial shrinkage of their tumors, which lasted an average of 23 months, compared to 3.9 percent of those taking Afinitor, lasting an average of 13.7 months.
Bovendien was het bijwerkingenprofiel voor nivolumab de helft van dat van everolimus 19% vs 37%.
De resultaten zijn ongeacht de status van de zogeheten PD-1/PD-L1 ligand expressie, die expressie zou noodzakelijk zijn voor de werking van nivolumab. Als patiënten daarop geselecteerd zouden zijn dan zouden de resultaten wellicht nog beter zijn geweest.
Alle reden dus om de tweedelijns behandeling (nog beter zou zijn nivolumab eerstelijns behandeling te maken) van gevorderde nierkanker te wijzingen lijkt mij.
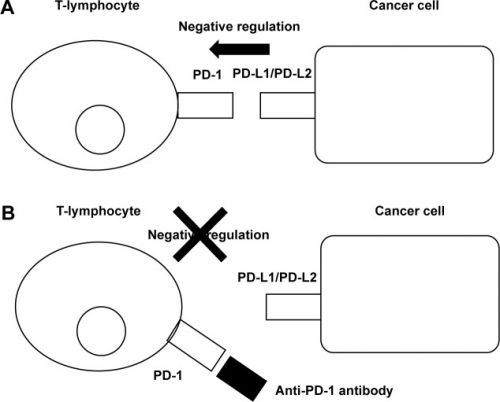
Grafiek: geeft werkingmechanisme weer van anti-PD medicijnen zoals Nivolumab - Obvido
Studieresultaten:
Totaal 821 patiënten met gevorderde nierkanker - clear-cell renal-cell carcinoma - die eerder 1 of twee keer waren behandeld met een behandeling met ook een angiogeneseremmer werden gerandomiseerd ingedeeld (in een 1:1 verhouding) om of 3 mg nivolumab per kilogram lichaamsgewicht te krijgen intraveneus elke 2 weken of een tablet met 10-mg everolimus tablet oraal een keer per dag in te nemen. Het primaire einddoel was overall overleving. Het tweede doel was de objecteive respons en de veiligheid - het bijwerkingenprofiel.
Statistisch significant betere resultaten voor nivolumab:
De median overall overleving was 25.0 maanden (95% confidence interval , 21.8 maanden tot nog niet bereikt) voor de groep die nivolumab kreeg en 19.6 maanden (95% CI, 17.6 tot 23.1 maanden) voor everolimus. De hazard ratio voor overlijden in de groep van de nivolumab versus everolimus was 0.73 (98.5% CI, 0.57 to 0.93; P=0.002), welke statistisch significant is (P≤0.0148).
De objectieve respons was ook beter voor nivolumab dan voor everolimus (25% versus. 5%; odds ratio, 5.98 [95% CI, 3.68 to 9.72]; P<0.001).
De mediane progressie-vrije overleving was 4.6 maanden (95% CI, 3.7 to 5.4) voor nivolumab en 4.4 maanden (95% CI, 3.7 to 5.5) voor everolimus (hazard ratio, 0.88; 95% CI, 0.75 to 1.03; P=0.11).
Graad 3 of 4 behandelings gerelateerde bijwerkingen deden zihc voor bij 19% van de patiënten uit de nivolumabgroep en bij 37% van de patiënten uit de everolimusgroep. De meest voorkomende bijwerking bij nivolumab was vermoeidheid (bij 2% van de patiënten), en de meest voorkomende bijerking bij everolimus was bloedarmoede (bij 8%).
Conclusie:
Conclusie van de onderzoekers: onder patiënten met vooraf behandelde gevorderde nierkanker - clear-cell renal carcinoma - bleek de overall overleving na behandeling met nivolumab significant langer en met minder bijwerkingen - graad 3/4 - gepaard gaande dan na een behandeling met everolimus.
Het volledige studierapport: Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. is gratis in te zien.
Het studieprotocol van de CheckMate 025 ClinicalTrials.gov is nummer, NCT01668784
Hier abstract van de studie zoals gepubliceerd in NEJM:
Among patients with previously treated advanced renal-cell carcinoma, overall survival was longer and fewer grade 3 or 4 adverse events occurred with nivolumab than with everolimus.
Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma.
Abstract
Supporting Efficacy Data
Approval was based on improved overall survival in a phase III trial (CheckMate 025) in which 821 patients with advanced renal cell carcinoma who had received prior antiangiogenic therapy were randomly assigned to nivolumab at 3 mg/kg intravenously every 2 weeks (n = 410) or oral everolimus (Afinitor) at 10 mg once daily (n = 411).2,3 Patients had a median age of 62 years (40% ≥ 65 and 9% ≥ 75 years), 75% were male, 88% were white, Karnofsky performance score was 70 to 80 in 34% and 90 to 100 in 66%, 77% had received prior antiangiogenic therapy, and Memorial Sloan Kettering risk was favorable in 34%, intermediate in 47%, and poor in 19%.
At a prespecified interim analysis based on 70% of planned events, median overall survival, the primary endpoint, was 25.0 months (95% confidence interval = 21.7 months to not estimable) in the nivolumab group vs 19.6 months (95% CI = 17.6–23.1 months) in the everolimus group (hazard ratio = 0.73, P = .0018). A survival benefit was observed regardless of programmed cell death ligand 1 (PD-L1) expression level. The confirmed response rate was 21.5% vs 3.9%, median response duration was 23.0 vs 13.7 months, and median time to response was 3.0 to 3.7 months
BACKGROUND:
Nivolumab, a programmed death 1 (PD-1) checkpoint inhibitor, was associated with encouraging overall survival in uncontrolled studies involving previously treated patients with advanced renal-cell carcinoma. This randomized, open-label, phase 3 study compared nivolumab with everolimus in patients with renal-cell carcinoma who had received previous treatment.
METHODS:
A total of 821 patients with advanced clear-cell renal-cell carcinoma for which they had received previous treatment with one or two regimens of antiangiogenic therapy were randomly assigned (in a 1:1 ratio) to receive 3 mg of nivolumab per kilogram of body weight intravenously every 2 weeks or a 10-mg everolimus tablet orally once daily. The primary end point was overall survival. The secondary end points included the objective response rate and safety.
RESULTS:
The median overall survival was 25.0 months (95% confidence interval , 21.8 to not estimable) with nivolumab and 19.6 months (95% CI, 17.6 to 23.1) with everolimus. The hazard ratio for death with nivolumab versus everolimus was 0.73 (98.5% CI, 0.57 to 0.93; P=0.002), which met the prespecified criterion for superiority (P≤0.0148). The objective response rate was greater with nivolumab than with everolimus (25% vs. 5%; odds ratio, 5.98 [95% CI, 3.68 to 9.72]; P<0.001). The median progression-free survival was 4.6 months (95% CI, 3.7 to 5.4) with nivolumab and 4.4 months (95% CI, 3.7 to 5.5) with everolimus (hazard ratio, 0.88; 95% CI, 0.75 to 1.03; P=0.11). Grade 3 or 4 treatment-related adverse events occurred in 19% of the patients receiving nivolumab and in 37% of the patients receiving everolimus; the most common event with nivolumab was fatigue (in 2% of the patients), and the most common event with everolimus was anemia (in 8%).
CONCLUSIONS:
Among patients with previously treated advanced renal-cell carcinoma, overall survival was longer and fewer grade 3 or 4 adverse events occurred with nivolumab than with everolimus. (Funded by Bristol-Myers Squibb; CheckMate 025 ClinicalTrials.gov number, NCT01668784.).
Comment in
- Renal-Cell Cancer--Targeting an Immune Checkpoint or Multiple Kinases. [N Engl J Med. 2015]
- PMID:
- 26406148
- [PubMed - indexed for MEDLINE]
-
References
-
1
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-E386
CrossRef | Web of Science | Medline
-
2
Fisher R, Gore M, Larkin J. Current and future systemic treatments for renal cell carcinoma. Semin Cancer Biol 2013;23:38-45
CrossRef | Web of Science | Medline
-
3
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: kidney cancer. Fort Washington, PA: National Comprehensive Cancer Network, 2015. Version 3.2015 (www.nccn.org/professionals/physician_gls/f_guidelines.asp).
-
4
Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:Suppl 3:iii49-iii56
CrossRef | Web of Science | Medline
-
5
Afinitor (everolimus) prescribing information. East Hanover, NJ: Novartis Pharmaceuticals, 2015 (www.pharma.us.novartis.com/product/pi/pdf/afinitor.pdf).
-
6
Dabney R, Devine R, Sein N, George B. New agents in renal cell carcinoma. Target Oncol 2014;9:183-193
CrossRef
-
7
Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007;104:3360-3365
CrossRef | Web of Science | Medline
-
8
Hamid O, Carvajal RD. Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther 2013;13:847-861
CrossRef | Web of Science | Medline
-
9
Nurieva RI, Liu X, Dong C. Molecular mechanisms of T-cell tolerance. Immunol Rev 2011;241:133-144
CrossRef | Web of Science | Medline
-
10
Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006;66:3381-3385
CrossRef | Web of Science | Medline
-
11
Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A 2004;101:17174-17179
CrossRef | Web of Science | Medline
-
12
Choueiri T, Fishman MN, Escudier B. Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma (mRCC): association of biomarkers with clinical outcomes. J Clin Oncol 2015;33:Suppl:4500. abstract.
Web of Science | Medline
-
13
Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-3175
CrossRef | Web of Science | Medline
-
14
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-2454
Free Full Text | Web of Science | Medline
-
15
Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma: results of a randomized phase II trial. J Clin Oncol 2015;33:1430-1437
CrossRef | Web of Science | Medline
-
16
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-247
CrossRef | Web of Science | Medline
-
17
Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187-193
Web of Science | Medline
-
18
Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 2004;22:454-463
CrossRef | Web of Science | Medline
-
19
Cogswell JP, Goldberg SM, Gupta AK, Jure-Kunkel M, Wang XT, Wigginton JM. Cancer immunotherapy by disrupting pd-1/pd-l1 signaling. FreePatentsOnline.com (http://www.freepatentsonline.com/20130309250.pdf).
-
20
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. 2010 (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf).
-
21
Cella D, Yount S, Brucker PS, et al. Development and validation of a scale to measure disease-related symptoms of kidney cancer. Value Health 2007;10:285-293
CrossRef | Web of Science | Medline
-
22
O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979;35:549-556
CrossRef | Web of Science | Medline
-
23
Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics 1982;38:29-41
CrossRef | Web of Science
-
24
Categorical data analysis. In: Berry DA, ed. Statistical methodology in the pharmaceutical sciences. New York: Marcel Dekker, 1990:391-475.
-
25
Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404-414
CrossRef | Web of Science
-
26
Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-330
Free Full Text | Web of Science | Medline
-
27
Paz-Ares L, Horn L, Borghaei H, et al. Phase III, randomized trial (CheckMate 057) of nivolumab (NIVO) versus docetaxel (DOC) in advanced non-squamous cell (non-SQ) non-small cell lung cancer (NSCLC). J Clin Oncol 2015;33:Suppl:LBA109. abstract.
Web of Science
-
28
Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med 2015;373:123-135
Full Text | Web of Science | Medline
-
29
Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers (Basel) 2011;3:3856-3893
CrossRef
-
30
Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271-2281
Free Full Text | Web of Science | Medline
-
31
Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013;14:552-562
CrossRef | Web of Science | Medline
-
32
Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1814-1823
Full Text
-
-
Gerelateerde artikelen
- Poeptransplantatie vergroot effectiviteit van immuuntherapie en vermindert ernstige bijwerkingen bij uitgezaaide gevorderde niercelkanker copy 1
- Specificiteit van het menselijk leukocyten antigeen (HLA) blijkt voorspellende biomarker voor effectiviteit van immuuntherapie met anti-PD medicijnen - checkpointremmers bij patienten met gevorderde niercelkanker
- Immuuntherapie met pembrolizumab geeft betere ziektevrije overleving (77 vs 68 procent) na operatie bij patiënten met nierkanker die een hoog risico liepen op recidief in vergelijking met een placebo
- Cabozantinib toegevoegd aan immuuntherapie met nivolumab en ipilimumab geeft betere ziekteprogressievrije tijd en ziektecontrole bij uitgezaaide onbehandelde nierkanker in vergelijking met placebo naast nivolumab en ipilimumab
- Immuuncheckpointremmers nivolumab + ipilimumab of pembrolizumab + axitinib geeft toch ziektecontrole en remissies in de klinische praktijk bij patiënten met gevorderde uitgezaaide nierkanker met een slechte prestatiestatus volgens ECOG PS ≥2
- Clostridium butyricum (CBM 588) een probioticum toegevoegd aan nivolumab plus ipilimumab verbeterd sterk de repons en progressievrije tijd voor patienten met uitgezaaide nierkanker
- Immuuntherapie met avelumab plus VEGF-remmer axitinib vermindert kans op recidief en meer ziektevrije overleving bij nierkankerpatienten (stadium III) na operatie
- Combinatiebehandelingen met vormen van immuuntherapie en anti-PD medicijnen geven veelbelovende resultaten bij (gevorderde) nierkanker. Hier de belangrijkste studies van afgelopen jaar
- Immuuntherapie met combinatiebehandeling van Nivolumab en cabozantinib als eerstelijnsbehandeling voor uitgezaaide nierkanker geeft veel betere resultaten dan stndaard behandeling sunitinib wat betreft progressievrije overleving en algehele overleving
- NLR meting - veranderende verhouding van neutrofielen tot lymfocyten - blijkt een uitstekende en eenvoudige manier om de werkzaamheid van immuuntherapie met anti-PD medicijnen tijdens behandelingsfase te controleren. copy 1
- Immuuntherapie met Atezolizumab plus Avestin - bevacizumab geeft betere progressievrije ziekte en overall overleving dan sunitinib bij patienten met gevorderde uitgezaaide nierkanker
- Avelumab plus Axitinib geeft veel betere progressievrije overleving (plus 5 maanden) dan sunitinib als eerstelijns behandeling bij nog niet behandelde uitgezaaide nierkanker.
- Pembrolizumab plus Axitinib geeft betere overleving op 1 jaar (plus 11 procent) en betere progressievrije ziekte dan met sunitinib voor nog niet behandelde uitgezaaide nierkanker
- Nivolumab (Obvio) alleen en samen met ipilimumab (Yervoy) geeft uitstekende resultaten bij gevorderde niercelkanker ook in vergelijking met sunitinib
- Nivolumab een immuuntherapeutisch anti PD medicijn geeft uitstekende resultaten bij vergevorderde nierkanker versus everolimus en krijgt van FDA goedkeuring voor gebruik als medicijn
- immuuntherapie met axitinib (Inlyta®) en pembrolizumab (Keytruda®) bij gevorderde nog onbehandelde nierkanker verdubbelt progressievrije ziekte (10 vs 20 maanden)
- Immuuntherapie met rocapuldencel-T (AGS-003), een vorm van dendritische celtherapie met individuele T-cel stimulatie geeft uitstekende resultaten bij nieuwe patienten met uitgezaaide nierkanker
- Immuuntherapie bij nierkanker, een overzicht



Plaats een reactie ...
Reageer op "Nivolumab een immuuntherapeutisch anti PD medicijn geeft uitstekende resultaten bij vergevorderde nierkanker versus everolimus en krijgt van FDA goedkeuring voor gebruik als medicijn"