5 januari 2017: lees ook dit artikel:
18 december 2021: Een recente studie over toepassing van PDT bij prostaatkanker (abstract staat onderaan artikel):
Prostate-specific membrane antigen (PSMA)-targeted photodynamic therapy enhances the delivery of PSMA-targeted magnetic nanoparticles to PSMA-expressing prostate tumors
Hier nog een wat oudere studie over PDT bij prostaatkanker:
Focal Therapy: A New Paradigm for the Treatment of Prostate Cancer
5 januari 2017: Bron: The Lancet 16 december 2016 online
PDT - Photo Dynamische Therapy gericht op in bloed circulerende tumorcellen vermindert sterk de progressie van lokale prostaatkanker bij patiënten die in een wait-and-see programma zaten in vergelijking met de patienten die geen PDT - Photo Dynamische Therapy kregen maar alleen het wait-and-see beleid volgden. Na 2 jaar bleek er in de PDT groep 58 (28%) van de 206 mannen in de PDT groep progressie van hun ziekte te vertonen tegenover 120 (58%) mannen van de 207 in de wait-and-see controlegroep (adjusted hazard ratio 0·34, 95% CI 0·24–0·46; p<0·0001)
Nog beter waren de cijfers voor een negatieve biopt 2 jaar na de PDT behandeling. 101 (49%) van de 206 mannen uit de PDT groep had een negatieve prostaat biopt vergeleken met 28 (14%) mannen van de 207 uit de wait-and-see controlegroep (adjusted risk ratio 3·67, 95% CI 2·53–5·33; p<0·0001)
Dit blijkt uit een gerandomiseerde Europese fase III studie in 47 ziekenhuizen (waaronder NKI / AvL Amsterdam en het Catharina Ziekenhuis Eindhoven) bij totaal 413 prostaatkankerpatienten. De studie is gepubliceerd in The Lancet Oncology, De studie is uitgevoerd onder leiding van de Franse Prof Abdel-Rahmène Azzouzi.
Onderstaand beeld is uit een andere studie met PDT - Photo Dynamische therapie op in bloed circulerende tumorcellen bij prostaatkankerpatienten. Tekst gaat onder dit beeld verder
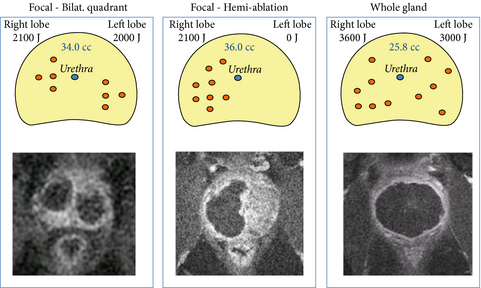
Schematic diagram of treatment plans and corresponding Day-7 MRI results. The three treatment plans for focal therapy of low-risk, early stage prostate cancer by WST11-mediated VTP therapy, which were considered by the Treatment Planning Guidance Committee, are displayed. The possible different fibre configurations used are shown.The red circles indicate the possible location of the fibres; the blue circle indicates the position of the urethra in the prostate gland. The corresponding MRIs, taken on Day-7, are shown underneath.
De studie van Prof. dr. Abdel-Rahmène Azzouzi en collega's werd uitgevoerd in de periode tussen maart 2011 en april 2013 bij zoals gezegd 413 prostaatkankerpatienten die in een wait-and-see beleid zaten. (Zie studieprotocol: https://clinicaltrials.gov/show/NCT01310894)
De PDT - Fotodynamische therapie bestond uit het gebruik van de fotosensitizer padeliporfin (TOOKAD® Soluble Vascular Targeted Photodynamic therapy (VTP)) in een dosis van 4 mg / kg intraveneus gedurende 10 minuten samen met optische vezels ingebracht in de prostaat in de behandelingszone. Daarna werd de behandelingszone belicht door laserlicht van 753 nm met een gefixeerde kracht van 150 mW/cm gedurende 22 minuten en 15 seconden.
Tekst gaat onder beeld verder
Bron foto: producent van padeliporfin
Met intervallen van drie maanden werd de PSA gemeten en vond daarnaast een digitale en een handmatige controle plaats. Op 12 maanden volgde een biopt. Na 2 jaar werden de studiersultaten geanalyseerd. Met dus meer dan uitstekende resultaten op ziekteprogressie en negaiteve biopten. Wel waren er wat meer bijwekeringen bij patienten uit de PDT groep maar die waren minimaal en goed behandelbaar.
Conclusie van de onderzoekers is dan ook dat PDT - Photo Dynamische Therapy gericht op de bloedvaten bij prostaatkankerpatienten in een wait-and-see beleid kan behoeden voor een prostaatverwijdering en verdere radicale behandeling.
Zie voor alle cijfers het abstract hieronder.
Kernpunten uit de studie:
Het volledige studierapport: Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial is tegen betaling in te zien of te downloaden.
Deze reviewstudie van PDT - Photo Dynamische Therapy op de in bloed circulrende tumrocellen : Vascular targeted photochemotherapy using padoporfin and padeliporfin as a method of the focal treatment of localised prostate cancer - clinician’s insight is wel als volledig studierapport in te zien.
Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
En als donateur kunt u ook korting krijgen bij verschillende bedrijven:
Hier in schema de studies die in deze review studie zijn geanalyseerd:
|
Published online 2016 Mar 26. doi: 10.5662/wjm.v6.i1.65
|
Table 2
Vascular targeted photodynamic therapy using padoporfin and padeliporfin in the treatment of localised prostate cancer - clinical trials
| Phase | No. of patients | Photosensitizer | Radiation | Ref. |
| I | 10 | Padoporfin, 0.1-2 mg/kg (0.1, 0.25, 1 and 2 mg/kg) | 763 nm, 100-360 J/cm | Weersink et al[83] |
| I | 24 | Padoporfin, 0.1-2 mg/kg | 763 nm, 100, 230 and 360 J/cm | Trachtenberg et al[56] |
| I/II | 15 | Padoporfin, 0.1-2 mg/kg | 763 nm, 100 J/cm | Gertner et al[84] |
| I/II | 34 | Padoporfin, 2 mg/kg | 763 nm, 100-300 J/cm | Arumainayagam et al[86] |
| I/II | 30 | Padeliporfin, 2, 4 and 6 mg/kg | 753 nm, 200 and 300 J/cm | https://clinicaltrials.gov/ct2/show/NCT00946881[87] |
| II | 28 | Padoporfin, 2 mg/kg | 763 nm, 0.1-1000 J/cm | Trachtenberg et al[57] |
| II | 40 | Padeliporfin, 2, 4 and 6 mg/kg | 753 nm, 200 J/cm | Arumainayagam et al[72] |
| II | 40 | Padeliporfin, 2-6 mg/kg | 753 nm, 200 J/cm | Quoraishi et al[74] |
| II | 85 | Padeliporfin, 4 mg/kg | 753 nm, 200 J/cm | Azzouzi et al[88] |
| II | 56 | Padeliporfin, 4 mg/kg | 753 nm, 200 J/cm | Eymerit-Morin et al[89] |
| II | 86 | Padeliporfin, 4 and 6 mg/kg | 753 nm, 200 and 300 J/cm | https://clinicaltrials.gov/ct2/show/-NCT00975429[90] |
| II | 117 | Padeliporfin, 4 mg/kg | 753 nm, 200 J/cm | Azzouzi et al[62] |
| II | 40 | Padeliporfin, 2, 4 and 6 mg/kg | 753 nm, 200 J/cm | Moore et al[67] |
| II | 40 | Padeliporfin, 2, 4 and 6 mg/kg | 753 nm, 200 and 300 J/cm | https://www.clinicaltrials.gov/ct2/show/NCT00707356[92] |
| II/III | 86 | Padeliporfin, 4 mg/kg | 753 nm, 200 J/cm | Azzouzi et al[91] |
| II/III | 16 | Padoporfin, 2 mg/kg | 763 nm, no information on radiation fluence | https://www.clinicaltrials.gov/ct2/show/-NCT00312442[93] |
| II/III | 1 | Padeliporfin, 4 mg/kg | 753 nm, 200 J/cm | Azzouzi et al[94] |
| II/III | 19 | Padeliporfin, 4 and 6 mg/kg | 753 nm, 200 and 300 J/cm | Lebdai et al[95] |
| III | 81 | Padeliporfin, 4 mg/kg | 753 nm, 200 J/cm | https://clinicaltrials.gov/ct2/show/-NCT01875393[96] |
| III | 400 | Padeliporfin, 4 mg/kg | 753 nm, 200 J/cm | https://clinicaltrials.gov/show/-NCT01310894[97] |
Hier de abstracten van de studies met een referentielijst.
Padeliporfin vascular-targeted photodynamic therapy is a safe, effective treatment for low-risk, localised prostate cancer. This treatment might allow more men to consider a tissue-preserving approach and defer or avoid radical therapy.
Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial
DOI: http://dx.doi.org/10.1016/S1470-2045(16)30661-1
Summary
Background
Vascular-targeted photodynamic therapy, a novel tissue-preserving treatment for low-risk prostate cancer, has shown favourable safety and efficacy results in single-arm phase 1 and 2 studies. We compared this treatment with the standard of care, active surveillance, in men with low-risk prostate cancer in a phase 3 trial.
Methods
This randomised controlled trial was done in 47 European university centres and community hospitals. Men with low-risk, localised prostate cancer (Gleason pattern 3) who had received no previous treatment were randomly assigned (1:1) to vascular-targeted photodynamic therapy (4 mg/kg padeliporfin intravenously over 10 min and optical fibres inserted into the prostate to cover the desired treatment zone and subsequent activation by laser light 753 nm with a fixed power of 150 mW/cm for 22 min 15 s) or active surveillance. Randomisation was done by a web-based allocation system stratified by centre with balanced blocks of two or four patients. Best practice for active surveillance at the time of study design was followed (ie, biopsy at 12-month intervals and prostate-specific antigen measurement and digital rectal examination at 3-month intervals). The co-primary endpoints were treatment failure (histological progression of cancer from low to moderate or high risk or death during 24 months' follow-up) and absence of definite cancer (absence of any histology result definitely positive for cancer at month 24). Analysis was by intention to treat. Treatment was open-label, but investigators assessing primary efficacy outcomes were masked to treatment allocation. This trial is registered with ClinicalTrials.gov, number NCT01310894.
Findings
Between March 8, 2011, and April 30, 2013, we randomly assigned 206 patients to vascular-targeted photodynamic therapy and 207 patients to active surveillance. Median follow-up was 24 months (IQR 24–25). The proportion of participants who had disease progression at month 24 was 58 (28%) of 206 in the vascular-targeted photodynamic therapy group compared with 120 (58%) of 207 in the active surveillance group (adjusted hazard ratio 0·34, 95% CI 0·24–0·46; p<0·0001). 101 (49%) men in the vascular-targeted photodynamic therapy group had a negative prostate biopsy result at 24 months post treatment compared with 28 (14%) men in the active surveillance group (adjusted risk ratio 3·67, 95% CI 2·53–5·33; p<0·0001). Vascular-targeted photodynamic therapy was well tolerated. The most common grade 3–4 adverse events were prostatitis (three [2%] in the vascular-targeted photodynamic therapy group vs one [<1%] in the active surveillance group), acute urinary retention (three [2%] vs one [<1%]) and erectile dysfunction (two [1%] vs three [1%]). The most common serious adverse event in the vascular-targeted photodynamic therapy group was retention of urine (15 patients; severe in three); this event resolved within 2 months in all patients. The most common serious adverse event in the active surveillance group was myocardial infarction (three patients).
Interpretation
Padeliporfin vascular-targeted photodynamic therapy is a safe, effective treatment for low-risk, localised prostate cancer. This treatment might allow more men to consider a tissue-preserving approach and defer or avoid radical therapy.
Funding
Steba Biotech.
References
- Mottet, N, Bellmunt, JE, van den Bergh, RCN et al. Guidelines on prostate cancer. European Association of Urology. http://uroweb.org/wp-content/uploads/EAU-Guidelines-Prostate-Cancer-2015-v2.pdf; 2015. ((accessed Nov 15, 2016).)
- Klotz, L, Vesprini, D, Sethukavalan, P et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015; 33: 272–277
- Klotz, L and Emberton, M. Management of low risk prostate cancer: active surveillance and focal therapy. Curr Opin Urol. 2014; 24: 270–279
- van den Bergh, RC, Ahmed, HU, Bangma, CH, Cooperberg, MR, Villers, A, and Parker, CC. Novel tools to improve patient selection and monitoring on active surveillance for low-risk prostate cancer: a systematic review. Eur Urol. 2014; 65: 1023–1031
- Summary
- | Full Text
- | Full Text PDF
- | PubMed
- | Scopus (55)
- Ahmed, HU, Hindley, RG, Dickinson, L et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol. 2012; 13: 622–632
- Summary
- | Full Text
- | Full Text PDF
- | PubMed
- | Scopus (166)
- Valerio, M, Ahmed, HU, Emberton, M et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur Urol. 2014; 66: 732–751
- Summary
- | Full Text
- | Full Text PDF
- | PubMed
- | Scopus (80)
- Azzouzi, AR, Barret, E, Bennet, J et al. TOOKAD® soluble focal therapy: pooled analysis of three phase II studies assessing the minimally invasive ablation of localized prostate cancer. World J Urol. 2015; 33: 945–953
- Crossref
- | PubMed
- | Scopus (8)
- Azzouzi, AR, Barret, E, Moore, CM et al. TOOKAD(®) Soluble vascular-targeted photodynamic (VTP) therapy: determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int. 2013; 112: 766–774
- Welty, CJ, Cowan, JE, Nguyen, H et al. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol. 2015; 193: 807–811
- Summary
- | Full Text
- | Full Text PDF
- | PubMed
- | Scopus (19)
- Heidenreich, A, Bellmunt, J, Bolla, M et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011; 59: 61–71
- Summary
- | Full Text
- | Full Text PDF
- | PubMed
- | Scopus (900)
- American Urological Association. Guideline for the management of clinically localized prostate cancer: 2007 update. http://www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer.pdf. ((accessed Nov 15, 2016).)
- Moore, CM, Azzouzi, AR, Barret, E et al. Determination of optimal drug dose and light dose index to achieve minimally invasive focal ablation of localised prostate cancer using WST11-vascular-targeted photodynamic (VTP) therapy. BJU Int. 2015; 116: 888–896
- Crossref
- | PubMed
- | Scopus (7)
- Azzouzi, AR, Lebdai, S, Benzaghou, F, and Stief, C. Vascular-targeted photodynamic therapy with TOOKAD® Soluble in localized prostate cancer: standardization of the procedure. World J Urol. 2015; 33: 937–944
- Barry, MJ, Fowler, FJ Jr, O'Leary, MP et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992; 148: 1549–1557
- Rosen, RC, Riley, A, Wagner, G, Osterloh, IH, Kirkpatrick, J, and Mishra, A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997; 49: 822–830
- Epstein, JI. A new contemporary prostate cancer grading system. Ann Pathol. 2015; 35: 474–476
- Panebianco, V, Barchetti, F, Sciarra, A et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol. 2015; 33: 17.e1–17.e7
- Summary
- | Full Text
- | Full Text PDF
- | PubMed
- | Scopus (35)
- Recabal, P, Assel, M, Sjoberg, DD et al. The efficacy of multiparametric magnetic resonance imaging and magnetic resonance imaging targeted biopsy in risk classification for patients with prostate cancer on active surveillance. J Urol. 2016; 196: 374–381
- Summary
- | Full Text
- | Full Text PDF
- | PubMed
- Jarow, JP, Ahmed, HU, Choyke, PL, Taneja, SS, and Scardino, PT. Partial gland ablation for prostate cancer: report of a Food and Drug Administration, American Urological Association, and Society of Urologic Oncology Public Workshop. Urology. 2016; 88: 8–13
- Summary
- | Full Text
- | Full Text PDF
- | PubMed
- Henderson, DR, de Souza, NM, Thomas, K et al. Nine-year follow-up for a study of diffusion-weighted magnetic resonance imaging in a prospective prostate cancer active surveillance cohort. Eur Urol. 2015; 69: 1028–1033
- Godtman, RA, Holmberg, E, Khatami, A, Pihl, CG, Stranne, J, and Hugosson, J. Long-term results of active surveillance in the Göteborg randomized, population-based prostate cancer screening trial. Eur Urol. 2016; 70: 760–766
- D'Amico, AV. Personalizing the use of active surveillance as an initial approach for men with newly diagnosed prostate cancer. J Clin Oncol. 2015; 33: 3365–3366
- Ahmed, HU, Berge, V, Bottomley, D et al. Can we deliver randomized trials of focal therapy in prostate cancer?. Nat Rev Clin Oncol. 2014; 11: 482–491
These results suggest that PSMA-targeted PDT enhances the delivery of PSMA-targeted MNPs to PSMA(+) tumors by enhancing the vascular permeability of the tumors.
Prostate-specific membrane antigen (PSMA)-targeted photodynamic therapy enhances the delivery of PSMA-targeted magnetic nanoparticles to PSMA-expressing prostate tumors
Associated Data
Abstract
Enhanced vascular permeability in tumors plays an essential role in nanoparticle delivery. Prostate-specific membrane antigen (PSMA) is overexpressed on the epithelium of aggressive prostate cancers (PCs). Here, we evaluated the feasibility of increasing the delivery of PSMA-targeted magnetic nanoparticles (MNPs) to tumors by enhancing vascular permeability in PSMA(+) PC tumors with PSMA-targeted photodynamic therapy (PDT).
Method: PSMA(+) PC3 PIP tumor-bearing mice were given a low-molecular-weight PSMA-targeted photosensitizer and treated with fluorescence image-guided PDT, 4 h after. The mice were then given a PSMA-targeted MNP immediately after PDT and monitored with fluorescence imaging and T2-weighted magnetic resonance imaging (T2-W MRI) 18 h, 42 h, and 66 h after MNP administration. Untreated PSMA(+) PC3 PIP tumor-bearing mice were used as negative controls.
Results: An 8-fold increase in the delivery of the PSMA-targeted MNPs was detected using T2-W MRI in the pretreated tumors 42 h after PDT, compared to untreated tumors. Additionally, T2-W MRIs revealed enhanced peripheral intra-tumoral delivery of the PSMA-targeted MNPs. That finding is in keeping with two-photon microscopy, which revealed higher vascular densities at the tumor periphery.
Conclusion: These results suggest that PSMA-targeted PDT enhances the delivery of PSMA-targeted MNPs to PSMA(+) tumors by enhancing the vascular permeability of the tumors.
Discussion
MNP-induced hyperthermia is currently being evaluated as a less morbid focal therapy for intermediate and high-risk localized prostate cancers 6, 7, 10. However, since the delivery of high concentrations of MNPs to tumors after intravenous administration is still a major challenge, current preclinical and clinical practices involve directly injecting the MNPs into tumors 6, 59. Direct injection can produce treatment-related morbidity, which can negatively impact the quality of life of patients post-treatment, and will miss metastatic foci 10.
Previously, we evaluated the feasibility of specifically enhancing the delivery of a PSMA-targeted MNP to PSMA(+) tumors in a preclinical dual PSMA(+) and PSMA(-) human prostate tumor mouse model, with high tumor vascular permeability 52. Tumor vascular permeability was modulated in that study by using large tumors (~250 mm3). In that study, we observed that although administering high MNP doses [50 mg/kg (30 mg of Fe/kg)] increased the amount of MNPs that accumulated in PSMA(+) tumors, it also increased the concentration of the MNPs that accumulated non-specifically in PSMA(-) tumors and the organs of the reticuloendothelial system (RES).
In this report, we evaluated a different strategy to enhance specifically the delivery of PSMA-targeted MNPs to PC tumors. We used our previously developed low-molecular-weight PSMA-targeted photosensitizer (YC-9) and a PDT pretreatment plan to enhance the vascular permeability of PSMA(+) tumors, with low tumor vascular permeability (~50 mm3), for the increased delivery of our previously developed PSMA-targeted MNPs [50 mg/kg (30 mg of Fe/kg)].
To estimate the contribution from the baseline tumor vascular permeability on MNP delivery in the PSMA(+) and PSMA(-) tumors, respectively, we estimated MNP delivery to the untreated PSMA(+) tumors (Group 3) and the untreated PSMA(-) tumors (Group 5), respectively. Using ex vivo fluorescence imaging (Table S3 and S5), we observed that the delivery of MNPs to the untreated PSMA(-) tumors (Group 5) was ~ 4.5-fold higher than to the untreated PSMA(+) tumors (Group 3). This suggested a higher contribution from the baseline vascular permeability in the PSMA(-) tumors compared to the PSMA(+) tumors. This difference was attributed to larger volumes of the PSMA(-) tumors than the PSMA(+) tumors. Thus, to take into account this baseline difference in the vascular permeability between the PSMA(+) and the PSMA(-) tumors in this study, direct comparisons were not made between the PDT pretreated PSMA(+), and the PDT pretreated PSMA(-) tumors. Instead, an indirect comparison was made as described below.
To evaluate the non-specific contributions from non-targeted PDT to enhance MNP delivery, tumors from PDT pretreated PSMA(-) PC3 flu (Group 4) mice were compared to those from untreated PSMA(-) PC3 flu (Group 5) mice. In vivo fluorescence and MRI results suggested a 3.6 ± 1.6-fold higher delivery of the MNPs in the PDT pretreated PSMA(-) tumors (Group 4), compared to the untreated PSMA(-) tumors (Group 5).
The contribution from PSMA-targeted PDT to enhance MNP delivery to tumors was next indirectly evaluated by comparing the difference between the PSMA(+) PC3 PIP groups versus that between the PSMA(-) PC3 flu groups [(Group 2 versus Group 3) compared to (Group 4 versus Group 5)]. From the in vivo fluorescence and MRI results, a 8.9 ± 2.6-fold higher delivery of the MNPs was detected in the PDT pretreated PSMA(+) tumors (Group 2), compared to the untreated PSMA(+) tumors (Group 3). Thus, by comparing the difference between the PSMA(+) groups to the difference between the PSMA(-) groups, the ~ 2-fold higher difference detected between the PSMA(+) groups was attributed to the specific contribution from PSMA-targeted PDT. These EPR effect enhancement values are comparable to those observed with other previously reported agents, designed for different tumor phenotypes 33, 42, 60.
Using the ex vivo fluorescence organ biodistribution ratios, we estimated that ~0.34 mg of iron/cm3 of the tumor volume was deposited in the PDT pretreated PSMA(+) tumors versus ~0.07 mg of iron/cm3 of the tumor volume in the non-treated PSMA(+) tumors. That iron concentration determined in the PDT-pretreated PSMA(+) tumors is higher than that reported to be required for efficient hyperthermia therapy (0.27 mg of iron/cm3 of the tumor volume), following direct tumor injections in rodents 59. However, the intra-tumoral MNP distribution was inhomogeneous. This could leave some tumor areas untreated and subsequently result in tumor regrowth and patient relapse 61, 62. Consequently, several strategies are currently being developed to homogenously enhance tumor vascular permeability and the delivery of MNPs to tumors 26, 61, 62. Furthermore, although this PDT pretreatment strategy was effective in enhancing the delivery of PSMA-targeted MNPs to PSMA(+) tumors with low tumor vascular permeability, there is still a need for the development and optimization of MNP delivery strategies to increase the delivery efficiency, by minimizing the accumulation of MNPs in organs of the RES, such as the liver.
Conclusion
We evaluated the feasibility of increasing the delivery of PSMA-targeted MNPs to PSMA(+) tumors with low tumor vascular permeability. Through the use of two complementary imaging techniques, we demonstrated that the delivery of PSMA-targeted MNPs to PSMA(+) tumors could be enhanced by PSMA-targeted PDT using a low-molecular-weight PSMA-targeted photosensitizer via the enhancement of tumor vascular permeability. MNP-induced hyperthermia and vascular-targeted PDT are both focal therapies currently being evaluated for the treatment of localized PC. Consequently, the use of low dose PSMA-targeted PDT to enhance the delivery of PSMA-targeted MNPs could contribute synergistically to effective long-term control of aggressive localized PC lesions. We anticipate that this strategy could be used to deliver drug-loaded, PSMA-targeted MNPs to localized, aggressive, PSMA-expressing, castration-resistant prostate tumors for enhanced MRI/MPI-guided hyperthermia, and sustained drug release.
Acknowledgments
We would like to thank Dr. Jiadi Xu and Ms. Kazi Akhter for their assistance with the MRI acquisitions. This research was sponsored by the National Institutes of Health (grant numbers: R01CA134675, U01CA183031, P50CA058236, P41EB024495, and R21HD097357).
Gerelateerde artikelen





Plaats een reactie ...
Reageer op "Prostaatkanker: PDT - Photo Dynamische Therapie op de bloedvaten bij prostaatkankerpatienten met een wait-and-see beleid geeft op 2 jaar verdubbeling 28% vs 58% van progressievrije ziekte in vergelijking met wait-and-see beleid"