Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
2 oktober 2022: Nieuwe studiegegevens van de phase III JAVELIN Bladder 100 trial bevestigen de goede resultaten van immuuntherapie met avelumab met statistisch significant langere algehele overleving (OS) met avelumab + best ondersteunende zorg (BSC) in vergelijking met best ondersteunende zorg (BSC) alleen bij patiënten met gevorderde urineleiderkanker - blaaskanker die geen ziekteprogressie hadden laten zien gedurende een 1L platinabevattende chemotherapie.
Hier de opeenvolgende resultaten uit deze fase III studie:
Zie dit studieverslag:
ASCO 2022: Long-Term Outcomes in Patients with Advanced Urothelial Carcinoma (UC) Who Received Avelumab First-Line Maintenance with or Without Second-Line Treatment: Exploratory Analyses from JAVELIN Bladder 100
10 mei 2017: Bron: FDA
Immuuntherapie met avelumab, een anti-PD medicijn, heeft van de FDA versnelde toelating gekregen voor gebruik bij gevorderde urineleiderkanker - blaaskanker stadium 4, nadat tijdens een tussenevaluatie uit een fase III studie: de phase III JAVELIN Bladder 100 trial goede resultaten werden gezien in progressievrije ziekte en overall overleving.
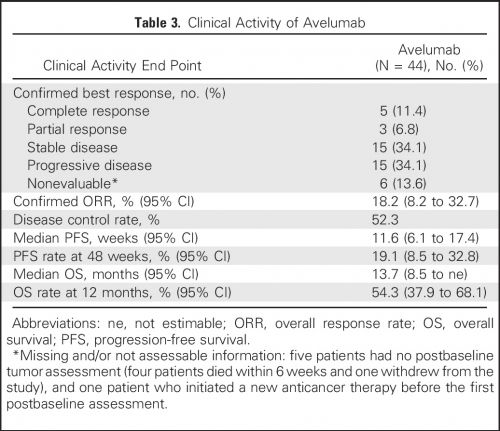
Overall response rate (ORR) bij patiënten (N = 242) met gevorderde urineleiderkanker die op z'n minst 13 weken de behandeling hebben gevolgd was 13.3% (n=30) (95% CI: 9.1, 18.4) en 16.1% (n=26) (95% CI: 10.8, 22.8) na een half jaar gebruik. Mediane tijd tot er een meetbare reactie werd gezien was 2.0 maanden (range 1.3-11.0). De mediane response duur is nog niet bereikt voor de patienten die minimaal 13 weken werden behandeld en / of die minimaal een half jaar werden behandeld. Tot nu toe bedraagt die (range: 1.4+ tot 17.4+ maanden in beide groepen.
Ik vertaal de resultaten nu niet verder in het Nederlands maar bekijk hieronder de grafieken en lees de FDA toelating die m.i. duidelijk genoeg zijn. Of gebruik de vertaaltool van google rechtsboven dit artikel.
Of bekijk deze andere studie met avelumab bij blaaskanker en urineleiderkanker: Avelumab, an Anti–Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study
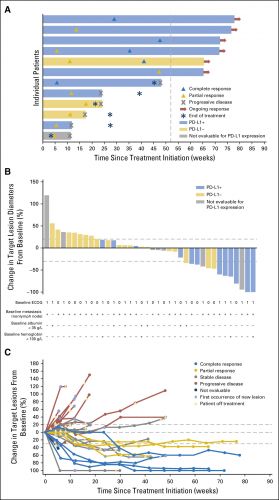
Hier de grafiek van de resultaten: (tekst loopt verder onder grafiek)
Het volledige studierapport: Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. is gratis in te zien.
Onderaan het abstract van de studie plus referentielijst
Hier de FDA toelating voor Aveluma:
FDA grants accelerated approval to avelumab for urothelial carcinoma
On May 9, 2017, the U.S. Food and Drug Administration granted accelerated approval to avelumab (BAVENCIO, EMD Serono, Inc.) for patients with locally advanced or metastatic urothelial carcinoma whose disease progressed during or following platinum-containing chemotherapy or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy.
Approval was based on data from an open-label, single arm, multi-center study that enrolled 242 patients with locally advanced or metastatic urothelial carcinoma whose disease progressed on or after platinum-based therapy or within 12 months of a platinum-containing neoadjuvant or adjuvant chemotherapy regimen. Patients received avelumab, 10 mg/kg intravenously, every 2 weeks until radiographic or clinical progression or unacceptable toxicity. All patients received pre-medication with an anti-histamine and acetaminophen prior to each avelumab administration. Confirmed overall response rate (ORR) in patients who had been followed for at least 13 weeks was 13.3% (n=30) (95% CI: 9.1, 18.4), and 16.1% (n=26) (95% CI: 10.8, 22.8) in patients who had been followed for at least 6 months. Median time to response was 2.0 months (range 1.3-11.0). The median response duration had not been reached in patients followed for at least 13 weeks or at least 6 months, but ranged from 1.4+ to 17.4+ months in both groups.
Avelumab was well tolerated and associated with durable responses and prolonged survival in patients with refractory metastatic Urothelial carcinoma of the bladder
DOI: 10.1200/JCO.2016.71.6795 Journal of Clinical Oncology - published online before print April 4, 2017
Avelumab, an Anti–Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study
Andrea B. Apolo, Jeffrey R. Infante, Ani Balmanoukian, Manish R. Patel, Ding Wang, Karen Kelly, Anthony E. Mega, Carolyn D. Britten, Alain Ravaud, Alain C. Mita, Howard Safran, Thomas E. Stinchcombe, Marko Srdanov, Arnold B. Gelb, Michael Schlichting, Kevin Chin, and James L. Gulley
Abstract
We assessed the safety and antitumor activity of avelumab, a fully human anti–programmed death-ligand 1 (PD-L1) IgG1 antibody, in patients with refractory metastatic urothelial carcinoma.
In this phase Ib, multicenter, expansion cohort, patients with urothelial carcinoma progressing after platinum-based chemotherapy and unselected for PD-L1 expression received avelumab 10 mg/kg intravenously every 2 weeks. The primary objectives were safety and tolerability. Secondary objectives included confirmed objective response rate (Response Evaluation Criteria in Solid Tumors version 1.1), progression-free survival, overall survival (OS), and PD-L1–associated clinical activity. PD-L1 positivity was defined as expression by immunohistochemistry on ≥ 5% of tumor cells.
Forty-four patients were treated with avelumab and followed for a median of 16.5 months (interquartile range, 15.8 to 16.7 months). The data cutoff was March 19, 2016. The most frequent treatment-related adverse events of any grade were fatigue/asthenia (31.8%), infusion-related reaction (20.5%), and nausea (11.4%). Grades 3 to 4 treatment-related adverse events occurred in three patients (6.8%) and included asthenia, AST elevation, creatine phosphokinase elevation, and decreased appetite. The confirmed objective response rate by independent central review was 18.2% (95% CI, 8.2% to 32.7%; five complete responses and three partial responses). The median duration of response was not reached (95% CI, 12.1 weeks to not estimable), and responses were ongoing in six patients (75.0%), including four of five complete responses. Seven of eight responding patients had PD-L1–positive tumors. The median progression-free survival was 11.6 weeks (95% CI, 6.1 to 17.4 weeks); the median OS was 13.7 months (95% CI, 8.5 months to not estimable), with a 12-month OS rate of 54.3% (95% CI, 37.9% to 68.1%).
Avelumab was well tolerated and associated with durable responses and prolonged survival in patients with refractory metastatic UC.
| 1. | Ervik M, Lam F, Ferlay J, et al: Cancer Today. Lyon, France: International Agency for Research on Cancer, 2016. http://gco.iarc.fr/today |
| 2. | Kaufman D, Raghavan D, Carducci M, et al: Phase II trial of gemcitabine plus cisplatin in patients with metastatic urothelial cancer. J Clin Oncol 18:1921-1927, 2000 Link |
| 3. | von der Maase H, Hansen SW, Roberts JT, et al: Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18:3068-3077, 2000 Link |
| 4. | Apolo AB, Ostrovnaya I, Halabi S, et al: Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst 105:499-503, 2013 CrossRef, Medline |
| 5. | Vaughn DJ, Broome CM, Hussain M, et al: Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol 20:937-940, 2002 Link |
| 6. | Galsky MD, Mironov S, Iasonos A, et al: Phase II trial of pemetrexed as second-line therapy in patients with metastatic urothelial carcinoma. Invest New Drugs 25:265-270, 2007 CrossRef, Medline |
| 7. | McCaffrey JA, Hilton S, Mazumdar M, et al: Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 15:1853-1857, 1997 Link |
| 8. | Bellmunt J, Théodore C, Demkov T, et al: Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 27:4454-4461, 2009 Link |
| 9. | Postow MA, Callahan MK, Wolchok JD: Immune checkpoint blockade in cancer therapy. J Clin Oncol 33:1974-1982, 2015 Link |
| 10. | TheraCys (BCG live), intravesical [package insert]. Toronto, Ontario, Canada: Sanofi Pasteur Limited; 2013. |
| 11. | Fuge O, Vasdev N, Allchorne P, et al: Immunotherapy for bladder cancer. Res Rep Urol 7:65-79, 2015 Medline |
| 12. | Alexandrov LB, Nik-Zainal S, Wedge DC, et al: Signatures of mutational processes in human cancer. Nature 500:415-421, 2013 CrossRef, Medline |
| 13. | Rizvi NA, Hellmann MD, Snyder A, et al: Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124-128, 2015 CrossRef, Medline |
| 14. | Chen DS, Mellman I: Oncology meets immunology: The cancer-immunity cycle. Immunity 39:1-10, 2013 CrossRef, Medline |
| 15. | Powles T, Eder JP, Fine GD, et al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515:558-562, 2014 CrossRef, Medline |
| 16. | Rosenberg JE, Hoffman-Censits J, Powles T, et al: Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 387:1909-1920, 2016 CrossRef, Medline |
| 17. | TECENTRIQ: (atezolizumab) injection [package insert]. South San Francisco, CA: Genentech, Inc; 2016. |
| 18. | McDaniel AS, Alva A, Zhan T, et al: Expression of PDL1 (B7-H1) before and after neoadjuvant chemotherapy in urothelial carcinoma. Eur Urol Focus 1:265-268, 2016 |
| 19. | Jiang L, Zhao Z, Jiang S, et al: Immunological markers predict the prognosis of patients with squamous non-small cell lung cancer. Immunol Res 62:316-324, 2015 CrossRef, Medline |
| 20. | Heery CR, O’Sullivan Coyne GH, Madan RA, et al: Phase I open-label, multiple ascending dose trial of MSB0010718C, an anti-PD-L1 monoclonal antibody, in advanced solid malignancies. J Clin Oncol 32:5s, 2014 (suppl; abstr 3064) |
| 21. | Grenga I, Donahue RN, Lepone LM, et al: A fully human IgG1 anti-PD-L1 MAb in an in vitro assay enhances antigen-specific T-cell responses. Clin Transl Immunology 5:e83, 2016 CrossRef, Medline |
| 22. | Vandeveer AJ, Fallon JK, Tighe R, et al: Systemic immunotherapy of non-muscle invasive mouse bladder cancer with avelumab, an anti-PD-L1 immune checkpoint inhibitor. Cancer Immunol Res 4:452-462, 2016 CrossRef, Medline |
| 23. | Boyerinas B, Jochems C, Fantini M, et al: Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res 3:1148-1157, 2015 CrossRef, Medline |
| 24. | Lepone LM, Donahue RN, Farsaci B, et al: Evaluation of immune cell subsets of cancer patients treated with a fully human IgG1 anti-PD-L1 MAb (MSB0010718C) capable of mediating ADCC of human tumor cells. Cancer Res 75:15 Suppl, 2015 (abstr 1316) |
| 25. | Eisenhauer EA, Therasse P, Bogaerts J, et al: New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 CrossRef, Medline |
| 26. | Agarwal N, Bellmunt J, Maughan BL, et al: Six-month progression-free survival as the primary endpoint to evaluate the activity of new agents as second-line therapy for advanced urothelial carcinoma. Clin Genitourin Cancer 12:130-137, 2014 CrossRef, Medline |
| 27. | Massard C, Gordon MS, Sharma S, et al: Safety and efficacy of durvalumab (MEDI4736), an anti–programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 34:3119-3125, 2016 Link |
| 28. | Sharma P, Callahan MK, Bono P, et al: Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): A multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 17:1590-1598, 2016 CrossRef, Medline |
| 29. | Plimack ER, Bellmunt J, Gupta S, et al: Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): A non-randomised, open-label, phase 1b study. Lancet Oncol 18:212-220, 2017 CrossRef, Medline |
Gerelateerde artikelen
- Atezolizumab als eerstelijnstherapie voor patiënten met uitgezaaide of lokaal gevorderde urineleiderkanker geeft betere overall overleving maar niet statistisch significant in vergelijking met chemotherapie
- Atezolizumab, een anti-PD medicijn geeft uitstekende resultaten bij uitgezaaide en zwaar voorbehandelde patienten met urineleiderkanker en blaaskanker
- nivolumab plus gemcitabine en cisplatine verbetert overall overleving van patienten met blaaskanker blijkt uit de checkmate 901 studie
- Immuuntherapie met Tislelizumab gecombineerd met Nab-Paclitaxel voor niet-spierinvasief urnieleider - blaaskanker met hoog risico geeft bijzonder goede resultaten
- C-reactief proteïne bloedwaarden (CRP) heeft betere voorspellende waarde voor aanslaan van immuuntherapie dan PD-L1 expressie bij patiënten met uitgezaaide urineleiderkanker - blaaskanker
- Immuuntherapie met maandelijkse vaste dosis durvalumab geeft duurzame remissies bij eerder met chemotherapie behandelde patiënten met gevorderde blaaskanker - urineleiderkanker
- Immuuntherapie met nivolumab geeft uitstekende resultaten bij gevorderde uitgezaaide blaaskanker - urineleiderkanker
- Circulerend tumor-DNA voorspelt respons op immuuntherapie met anti-PD medicijn Atezolizumab bij spierinvasieve urineleiderkanker en blaaskanker
- Hoe eerder na chemo gestart met Avelumab, een immuuntherapeutisch medicijn als onderhoudsbehandeling,hoe beter de resultaten op ziektevrije tijd en overall overleving bij patienten met inoperabele blaaskanker en urineleiderkanker
- Immuuntherapie met gemoduleerd verkoudheidsvirus (Coxsackievirus A21) vooraf aan operatie van blaaskanker is succesvol
- Pembrolizumab - Keytruda - anti PD medicijn - geeft uitstekende resultaten bij gevorderde blaaskanker blijkt uit follow-up gegevens van eerder wegens succes stopgezette studie.
- Avelumab, een anti-PD medicijn door FDA goedgekeurd voor gebruik na falende chemo bij gevorderde urineleiderkanker - blaaskanker stadium 4
- Immuuntherapie met nivolumab en ipilimumab samen geeft hoopgevende resultaten bij zwaar voorbehandelde gevorderde uitgezaaide blaaskanker
- BCG - Bacillus Calmette-Guerin bij blaaskanker: Hier een mini overzicht gepubliceerd van wetenschappelijke studies en bewijzen over het gebruik van BCG - Bacillus Calmette-Gue´rin - als succesvolle immuuntherapie bij blaaskanker. Update 23 februari 2010
- Immuuntherapie bij blaaskanker: een overzicht inclusief immuuntherapie met anti-PD medicijnen - checkpointremmers



Plaats een reactie ...
Reageer op "Avelumab, een anti-PD medicijn door FDA goedgekeurd voor gebruik na falende chemo bij gevorderde urineleiderkanker - blaaskanker stadium 4"