Aan dit artikel is uren gewerkt. Opzoeken van gerelateerde informatie, vertalen enz. Mocht u ons willen helpen om kanker-actueel online te houden en verder uit te breiden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
2 juni 2017: Bronnen: Phytomedicine, en Journal of Traditional and Complementary Medicine
Ik denk dat wel algemeen bekend is dat soja en genisteine vormen van hormoon gerelateerde kanker, waaronder borstkanker, prostaatkanker, eierstokkanker en endometriose kan remmen en voorkomen.Zie anders onze studielijsten: https://kanker-actueel.nl/NL/literatuurlijsten-niet-toxische-middelen-en-behandelingen-per-kankersoort-en-aanvullend-op-chemo-operatie-en-bestraling.html
Hier twee studies die bewijzen dat genisteine de klachten van endometriose kan verminderen en kan voorkomen dat endometriose uitgroeit tot kanker. En ook een reviewstudie van studies die aantonen dat genisteine eierstokkanker kan voorkomen en wellicht kan afremmen en recidief langer kan uitstellen.
In deze kleinschalige studie: Genistein aglycone: A new therapeutic approach to reduce endometrial hyperplasia verminderde na 6 maanden bij 42% van de patienten die genistein aglyconegebruikten de symptomen / klachten (histologische bevestiging van 29%) vergeleken met 47% bij patiënten die norethisterone acetate gebruikten (histologischge bevestiging van 31%), maar slechts bij 12% in de placebogroep waarbij bij 19% uit die groep de kalchten verergerden met 19 procent en de endometriose alleen maar erger werd. Zie verder abstract hieronder.
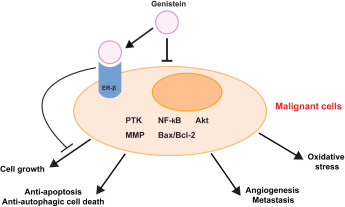
In deze reviewstudie: Genistein as a Potential Anticancer Agent against Ovarian Cancer wordt een overzicht gegeven van een groot aantal studies en daaruit blijkt dat genisteine effect heeft op bepaalde mutaties en eiwitexpressies en 'pathways' behorend bij vormen van hormoongerelateerde vormen van kanker waaronder met name eierstokkanker. Hier de conclusie uit de reviewstudie die ik niet verder vertaal maar anders vertaalt u dit met hulp van google vertaaltool rechtsboven dit artikel.
Conclusie:
Collectively, previous epidemiological and experimental studies have suggested that genistein may play promising roles as a chemopreventive or therapeutic agent against ovarian cancer. In addition to its hormonal action, the anticancer effects of genistein are related to multiple cellular signaling pathways such as PTK, Akt, NF-κB, MMP, and Bax/Bcl-2. Because the successful treatment of ovarian cancer is limited mainly by the development of chemotherapy resistance, genistein is expected to play a role in sensitizing multiresistant ovarian cancer cells and have additive effects with conventional chemotherapeutic agents. Although some clinical trials are now being performed to identify the role of genistein as an anticancer agent against various types of cancers, there is currently no registered clinical trial on ovarian cancer. Further research should be performed to identify the role of genistein in ovarian carcinogenesis.
Hier enkele grafieken uit deze studie van opgenomen studies in deze reviewstudie (klik op de blauwe links in de kolom refences om een bepaalde studie in te zien. Onderaan het abstract en referentielijst oinderaan dit artikel.
-
Table 1.
Effects of genistein on ovarian cancer development from different epidemiological studies
-
Reference Study design Study period Participants Race/Country Measure of soy intake Adjusted OR (95% CI) Zhang et al., 2004 CCS 1999–2000 254 EOC cases and 652 age-matched controls Chinese/China Soy foods (g/day): ≥ 136.4 vs. ≤ 47.0
Isoflavones (mg/day): ≥ 32.8 vs. ≤ 11.6
Genistein (mg/day): ≥ 20.9 vs. ≤ 6.60.50 (0.31–0.82)
0.51 (0.31–0.85)
0.50 (0.30–0.84)Sakauchi et al., 2007 PCS 1988–2003(15) 77 EOC cases among 63,541 women Japanese/Japan Soybean curd (times/week)
Almost every day vs. ≤ 1–20.61
(0.26–1.45)Rossi et al., 2008 CCS 1992–1997 1031 EOC cases and 2411 controls Italian/Italy Isoflavone (μg/day)
≥ 32.5 vs. ≤ 12.80.51
(0.37–0.69)Chang et al., 2007 PCS 1995–2003(8) 280 EOC cases among 97,275 women Mainly white (84%), Hispanic (4%), Asian (3%), black (2%), Native American (2%)/U.S. Isoflavone (mg/day) ≥ 3 vs. ≤ 1
Genistein (mg/day) ≥ 1.1 vs. ≤ 0.30.56 (0.33–0.96)
0.65 (0.42–1.02)Bandera et al., 2011 CCS 2004–2008 205 EOC cases and 390 controls White (87%), black (4%), Hispanic (4%)/U.S. Total phytoestrogen (mg/1000 kcal)
≥ 1395 vs. ≤ 5340.62
(0.38–1.00)Hedelin et al., 2011 PCS 1991–2007(16) 163 EOC cases (117 invasive cancer, 6 BT) among 47,140 women Swedish/Sweden Isoflavone (μg/day)
≥ 16 vs. ≤ 0.741.26
(0.79–2.01) -
CCS: case-control study, PCS: prospective cohort study, EOC: epithelial ovarian cancer, BT: borderline tumor
In vitro Studies
Results from previous epidemiological studies have raised the interest regarding the role of genistein as a chemopreventive agent, and therefore, researchers have attempted to determine the molecular mechanisms of this compound. Numerous studies have shown that genistein has inhibitory effects on ovarian cancer cells and tumor growth in vitro. Table 2 shows the anticancer effects of genistein on ovarian cancer cells from recently published in vitro studies.
-
Table 2.
Effects of genistein on ovarian cancer cell lines from in vitro studies
-
Reference Materials Cell type Description Measure of interest Results Choi et al., 2007 Genistein SK-OV-3 Human ovarian cancer cell Cell proliferation (MTT assay)
Cell cycle distribution (FACS)
Cytotoxicity (LDH)
Apoptosis (caspase-3 activity)Inhibition of cell proliferation in a dose- and time-dependent manner
Arrest at G2/M phase
Increased LDH release
Increased caspase-3 activityAhmed et al., 2011 ITB-301, genistein derivative SK-OV-3, ES2, HeyA8, HeyA8-MDR Human ovarian cancer cell Cell proliferation (crystal violet staining)
Cell cycle distribution (FACS)
Apoptosis (caspase-3/7 activity)
Microtubule depolymerization (tubulin polymerization assay kit)Induced microtubule depolymerization in a dose- and time-dependent manner
No change in efflux-mediated drug resistance
Cytotoxic effect of ITB-301 on a multidrug-resistant cell lineSolomon et al., 2008 Genistein A2780, C200 Human ovarian cancer cell Cell survival and apoptosis
(MTT assay, histone-DNA ELISA)
Indirect measure of apoptosis:
Bcl-2, Bcl-xL, c-IAP1, survivin
(Western blot analysis)
NF-κB (EMSA)Downregulation of antiapoptotic genes
Decreased NF-κB
Genistein pretreatment with chemotherapy was effective in both PS and PR ovarian cancer cell linesGercel-Taylor et al., 2004 Genistein 5 ovarian cancer cell lines from stage IIIc Human ovarian cancer cell Cell growth (sulforhodamine B and colony formation assays)
Apoptosis (caspase-3 activity)Induced caspase-3 activity
Additive effect of cell proliferation inhibition with cisplatin, topotecan, and paclitaxelLuo et al., 2008 Genistein OVCAR-3 Human ovarian cancer cell Cytotoxicity (CytoTox 96)
Proliferation (CellTiter 96)
VEGF (RT-PCR, ELISA)Induced cell growth
Inhibited VEGF expressionRucinska et al., 2007 G8CG CHO Chinese hamster ovary cell Cytotoxicity (MTT assay)
DNA damage (Comet assay)
Apoptosis
(Hoechst 33258/propidium iodide staining technique)
ROS (fluorescence probe)Cytotoxic at high concentrations
Antioxidant properties at lower concentrationsChen et al., 2001 Genistein Caov-3, NIH: OVCAR-3 Human ovarian cancer cell Cell proliferation (MTT assay))
ER, GAPDH (RT-PCR))
IL-6 (ELISA))
TGF-b (immunoassay)Inhibited cell proliferation
Inhibited IL-6 synthesis
Increased TGF-b productionGossner et al., 2007 Genistein A2780, CaOV3, ES2 Human ovarian cancer cell Analysis of apoptosis (FACS)
Autophagy (microtubule-associated LC3)
Akt (western blot analysis), glucose uptakeInduced apoptosis
Inhibited glucose uptake, reduced phosphorylated Akt
Induced autophagic cell death -
MTT: methyl thiazolyl tetrazolium, FACS: fluorescence-activated cell sorting, LDH: lactate dehydrogenase, ELISA: enzyme-linked immunosorbent assay, EMSA: electrophoretic mobility shift assay, PS: platinum sensitive, PR: platinum resistant, VEGF: vascular endothelial growth factor, RT-PCR: reverse transcription polymerase chain reaction, G8CG: genistein 8-C-glucoside, ROS: reactive oxygen species, ER: estrogen receptor, GADPH: glyceraldehyde-3-phosphate dehydrogenase, LC3: light chain 3
Hier de respectievelijke abstracten en referentielijst:
genistein aglycone might be useful for the management of endometrial hyperplasia without atypia in women that cannot be treated with progestin.
Phytomedicine
Genistein aglycone: A new therapeutic approach to reduce endometrial hyperplasia
- a
- Department of Clinical and Experimental Medicine and Pharmacology, Section of Pharmacology, Torre Biologica 5th floor, c/o AOU Policlinico G. Martino, Via C. Valeria, Gazzi, 98125, Messina, Italy
- b
- Department of Obstetrical and Gynaecological Sciences, University of Messina, Building A, c/o AOU Policlinico G. Martino, Via C. Valeria, Gazzi, 98125, Italy
- c
- Department of Human Pathology, University of Messina, Building D, c/o AOU Policlinico G. Martino, Via C. Valeria, Gazzi, 98125, Messina, Italy
- d
- Department of Biochemical, Physiological and Nutritional Sciences, Section of Physiology and Human Nutrition, University of Messina, Torre Biologica 5th floor, c/o AOU Policlinico G. Martino, Via C. Valeria, Gazzi, 98125, Messina, Italy
Collectively, previous epidemiological and experimental studies have suggested that genistein may play promising roles as a chemopreventive or therapeutic agent against ovarian cancer. In addition to its hormonal action, the anticancer effects of genistein are related to multiple cellular signaling pathways such as PTK, Akt, NF-κB, MMP, and Bax/Bcl-2.
Volume 2, Issue 2, April–June 2012, Pages 96–104
Genistein as a Potential Anticancer Agent against Ovarian Cancer
- Open Access funded by Center for Food and Biomolecules, National Taiwan University
- Under a Creative Commons license
Abstract
Genistein is known as the major component of isoflavone, which is present in high-soy diets. Genistein has received much attention because of its chemopreventive and therapeutic effects on various types of cancers. Numerous studies have shown that genistein has antineoplastic effects against ovarian cancer. Several epidemiological studies have shown that women who have high consumption of isoflavones have a relatively low incidence of ovarian cancer. Genistein inhibits ovarian carcinogenesis by pleiotropic mechanisms. A higher affinity to estrogen receptor β is one probable explanation for its ability to reduce the risk of ovarian cancer. Genistein also targets multiple cellular signal transduction pathways associated with cell cycle regulation and apoptosis. In addition, genistein has been suggested to have antiangiogenic and antioxidant activities. Herein, we summarize recent results from epidemiological and experimental studies to identify the role of genistein in ovarian cancer. Further studies are needed to achieve conclusive results and determine the clinical applications of genistein.
References
-
- Adlercreutz et al., 1993
-
Plasma concentrations of phyto-oestrogens in Japanese men
-
Lancet, 342 (1993), pp. 1209–1210
- | | |
-
- Ahmed et al., 2011
-
A genistein derivative, ITB-301, induces microtubule depolymerization and mitotic arrest in multidrug-resistant ovarian cancer
-
Cancer Chemotherapy and Pharmacology, 68 (2011), pp. 1033–1044
- | |
-
- Akiyama et al., 1987
-
Genistein, a specific inhibitor of tyrosine-specific protein kinases
-
Journal of Biological Chemistry, 262 (1987), pp. 5592–5595
- |
-
- Allred et al., 2001
-
Soy diets containing varying amounts of genistein stimulate growth of estrogen-dependent (MCF-7) tumors in a dose-dependent manner
-
Cancer Research, 61 (2001), pp. 5045–5050
- |
-
- Ames et al., 1995
-
The causes and prevention of cancer
-
Proceedings of the National Academy of Sciences of the United States of America, 92 (1995), pp. 5258–5265
- | |
-
- Andres et al., 2011
-
Risks and benefits of dietary isoflavones for cancer
-
Critical Reviews in Toxicology, 41 (2011), pp. 463–506
- | |
-
- Bandera et al., 2011
-
Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study
-
BMC Women’s Health, 11 (2011), p. 40
-
- Banerjee et al., 2008
-
Multi-targeted therapy of cancer by genistein
-
Cancer Letters, 269 (2008), pp. 226–242
- | | |
-
- Brandenberger et al., 1998
-
Estrogen receptor alpha (ER-alpha) and beta (ER-beta) mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: down-regulation of ER-beta in neoplastic tissues
-
Journal of Clinical Endocrinology and Metabolism, 83 (1998), pp. 1025–1028
- | |
-
- Brunet et al., 1999
-
Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor
-
Cell, 96 (1999), pp. 857–868
- | | |
-
- Cardone et al., 1998
-
Regulation of cell death protease caspase-9 by phosphorylation
-
Science, 282 (1998), pp. 1318–1321
- | |
-
- Carmeliet and Jain, 2000
-
Angiogenesis in cancer and other diseases
-
Nature, 407 (2000), pp. 249–257
- | |
-
- Chang et al., 2007
-
Diet and risk of ovarian cancer in the California Teachers Study cohort
-
American Journal of Epidemiology, 165 (2007), pp. 802–813
- | |
-
- Chen and Anderson, 2001
-
Isoflavones inhibit proliferation of ovarian cancer cells in vitro via an estrogen receptor-dependent pathway
-
Nutrition and Cancer, 41 (2001), pp. 165–171
- | |
-
- Choi et al., 2007
-
Pro-apoptotic effect and cytotoxicity of genistein and genistin in human ovarian cancer SK-OV-3 cells
-
Life Science, 80 (2007), pp. 1403–1408
- | | |
-
- Chu et al., 2000
-
Estrogen receptor isoform gene expression in ovarian stromal and epithelial tumors
-
Journal of Clinical Endocrinology and Metabolism, 85 (2000), pp. 1200–1205
- | |
-
- Couse et al., 1997
-
Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse
-
Endocrinology, 138 (1997), pp. 4613–4621
- | |
-
- Davis et al., 2001
-
Soy isoflavone supplementation in healthy men prevents NF-kappa B activation by TNF-alpha in blood lymphocytes
-
Free Radical Biology and Medicine, 30 (2001), pp. 1293–1302
- | | |
-
- De Wilde et al., 2007
-
Dietary isoflavones act on bone marrow osteoprogenitor cells and stimulate ovary development before influencing bone mass in pre-pubertal piglets
-
Journal of Physiological Sciences, 212 (2007), pp. 51–59
- | |
-
- Diel et al., 2006
-
Combinatorial effects of the phytoestrogen genistein and of estradiol in uterus and liver of female Wistar rats
-
Journal of Steroid Biochemistry and Molecular Biology, 102 (2006), pp. 60–70
- | | |
-
- Dorward et al., 2007
-
LH analog and dietary isoflavones support ovarian granulosa cell tumor development in a spontaneous mouse model
-
Endocrine-Related Cancer, 14 (2007), pp. 369–379
- | |
-
- Dreher and Junod, 1996
-
Role of oxygen free radicals in cancer development
-
European Journal of Cancer Prevention, 32 (1996), pp. 30–38
- | | |
-
- Elstrom et al., 2004
-
Akt stimulates aerobic glycolysis in cancer cells
-
Cancer Research, 64 (2004), pp. 3892–3899
- | |
-
- Fathalla, 1971
-
Incessant ovulation--a factor in ovarian neoplasia?
-
Lancet, 2 (1971), p. 163
- | | |
-
- Fotsis et al., 1995
-
Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro angiogenesis
-
Journal of Nutrition, 125 (1995), pp. 790–797
-
- Gercel-Taylor et al., 2004
-
Inhibitory effect of genistein and daidzein on ovarian cancer cell growth
-
Anticancer Research, 24 (2004), pp. 795–800
- |
-
- Gossner et al., 2007
-
Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells
-
Gynecologic Oncology, 105 (2007), pp. 23–30
- | | |
-
- Harris et al., 2005
-
Phytoestrogens induce differential estrogen receptor alpha- or Beta-mediated responses in transfected breast cancer cells
-
Experimental Biology and Medicine (Maywood), 230 (2005), pp. 558–568
- | |
-
- Hedelin et al., 2011
-
Dietary phytoestrogens and the risk of ovarian cancer in the women’s lifestyle and health cohort study
-
Cancer Epidemiology, Biomarkers & Prevention, 20 (2011), pp. 308–317
- | |
-
- Hernandez-Montes et al., 2006
-
Activation of glutathione peroxidase via Nrf1 mediates genistein’s protection against oxidative endothelial cell injury
-
Biochemical and Biophysical Research Communications, 346 (2006), pp. 851–859
- | | |
-
- Kasiske et al., 1991
-
Impact of dietary fatty acid supplementation on renal injury in obese Zucker rats
-
Kidney International, 39 (1991), pp. 1125–1134
- | | | |
-
- Kim et al., 2011
-
Modulation of inflammatory signaling pathways by phytochemicals in ovarian cancer
-
Genes and Nutrition, 6 (2011), pp. 109–115
- | |
-
- Kuiper et al., 1998
-
Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta
-
Endocrinology, 139 (1998), pp. 4252–4263
- | |
-
- Kyle et al., 1997
-
Genistein-induced apoptosis of prostate cancer cells is preceded by a specific decrease in focal adhesion kinase activity
-
Molecular Pharmacology, 51 (1997), pp. 193–200
- |
-
- Lee et al., 1991
-
Dietary effects on breast-cancer risk in Singapore
-
Lancet, 337 (1991), pp. 1197–1200
- | | |
-
- Lee et al., 2003
-
Soy and isoflavone consumption in relation to prostate cancer risk in China
-
Cancer Epidemiology, Biomarkers and Prevention, 12 (2003), pp. 665–668
- |
-
- Li et al., 1999
-
Induction of apoptosis and inhibition of c-erbB-2 in MDA-MB-435 cells by genistein
-
International Journal of Oncology, 15 (1999), pp. 525–533
- |
-
- Li and Sarkar, 2002
-
Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway
-
Clinical Cancer Research, 8 (2002), pp. 2369–2377
- |
-
- Lian et al., 2001
-
Preventive effects of isoflavones, genistein and daidzein, on estradiol-17beta-related endometrial carcinogenesis in mice
-
Japanese Journal of Cancer Research, 92 (2001), pp. 726–734
- | |
-
- Luo et al., 2008
-
Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids
-
Nutrition and Cancer, 60 (2008), pp. 800–809
- | |
-
- Magee and Rowland, 2004
-
Phyto-oestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer
-
British Journal of Nutrition, 91 (2004), pp. 513–531
- | |
-
- Mosselman et al., 1996
-
ER beta: identification and characterization of a novel human estrogen receptor
-
FEBS Letters, 392 (1996), pp. 49–53
- | | | |
-
- Myung et al., 2009
-
Soy intake and risk of endocrine-related gynaecological cancer: a meta-analysis
-
British Journal of Obstetrics and Gynaecology, 116 (2009), pp. 1697–1705
- | |
-
- Ness and Cottreau, 1999
-
Possible role of ovarian epithelial inflammation in ovarian cancer
-
Journal of the National Cancer Institute, 91 (1999), pp. 1459–1467
- | |
-
- Oesterreich et al., 2001
-
Re-expression of estrogen receptor alpha in estrogen receptor alpha-negative MCF-7 cells restores both estrogen and insulin-like growth factor-mediated signaling and growth
-
Cancer Research, 61 (2001), pp. 5771–5777
- |
-
- Ouyang et al., 2009
-
Genistein induces G2/M cell cycle arrest and apoptosis of human ovarian cancer cells via activation of DNA damage checkpoint pathways
-
Cell Biology International, 33 (2009), pp. 1237–1244
- | | |
-
- Ozes et al., 1999
-
NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase
-
Nature, 401 (1999), pp. 82–85
- |
-
- Parkin et al., 1999
-
Global cancer statistics
-
CA: A Cancer Journal of Clinicians, 49 (33–64) (1999), p. 31
-
- Peeters et al., 2003
-
Phytoestrogens and breast cancer risk. Review of the epidemiological evidence
-
Breast Cancer Research and Treatment, 77 (2003), pp. 171–183
- | |
-
- Pike et al., 1999
-
Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist
-
EMBO Journal, 18 (1999), pp. 4608–4618
- | |
-
- Rimoldi et al., 2007
-
Effects of chronic genistein treatment in mammary gland, uterus, and vagina
-
Environmental Health Perspectives, 115 (2007), pp. 62–68
- | |
-
- Romashkova and Makarov, 1999
-
NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling
-
Nature, 401 (1999), pp. 86–90
- |
-
- Rossi et al., 2008
-
Flavonoids and ovarian cancer risk: A case-control study in Italy
-
International Journal of Cancer, 123 (2008), pp. 895–898
- | |
-
- Rucinska et al., 2007
-
Effect of the phytoestrogen, genistein-8-C-glucoside, on Chinese hamster ovary cells in vitro
-
Cell Biology International, 31 (2007), pp. 1371–1378
- | | |
-
- Ruiz-Larrea et al., 1997
-
Antioxidant activity of phytoestrogenic isoflavones
-
Free Radical Research, 26 (1997), pp. 63–70
- | |
-
- Sakauchi et al., 2007
-
Dietary habits and risk of ovarian cancer death in a large-scale cohort study (JACC study) in Japan
-
Nutrtion and Cancer, 57 (2007), pp. 138–145
- | |
-
- Sarkar and Li, 2002
-
Mechanisms of cancer chemoprevention by soy isoflavone genistein
-
Cancer Metastasis Reviews, 21 (2002), pp. 265–280
- | |
-
- Semenza et al., 2001
-
‘The metabolism of tumours′: 70 years later
-
Novartis Foundation Symposium, 240 (2001), pp. 251–260
- |
-
- Seo et al., 2006
-
Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha
-
Breast Cancer Research and Treatment, 99 (2006), pp. 121–134
- | |
-
- Solomon et al., 2008
-
Sensitization of ovarian cancer cells to cisplatin by genistein: the role of NF-kappaB
-
Journal of Ovarian Research, 1 (2008), p. 9
- | |
-
- Spinozzi et al., 1994
-
The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkat T-leukemia cells
-
Leukemia Research, 18 (1994), pp. 431–439
- | | |
-
- Stadel, 1975
-
Letter: The etiology and prevention of ovarian cancer
-
American Journal of Obstetrics and Gynecology, 123 (1975), pp. 772–774
- | | |
-
- Strom et al., 2004
-
Estrogen receptor beta inhibits 17beta-estradiol-stimulated proliferation of the breast cancer cell line T47D
-
Proceedings of the National Academy of Sciences of the United States of America, 101 (2004), pp. 1566–1571
- | |
-
- Tanaka et al., 2002
-
Inhibitory effects of estrogenic compounds, 4-nonylphenol and genistein, on 7,12-dimethylbenz[a]anthracene-induced ovarian carcinogenesis in rats
-
Ecotoxicology and Environmental Safety, 52 (2002), pp. 38–45
- | | |
-
- Taylor et al., 2009
-
The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies
-
Nutrition Reviews, 67 (2009), pp. 398–415
- | |
-
- Treeck et al., 2007
-
Estrogen receptor {beta}1 exerts antitumoral effects on SK-OV-3 ovarian cancer cells
-
Journal of Endocrinology, 193 (2007), pp. 421–433
- | |
-
- Trock et al., 2006
-
Meta-analysis of soy intake and breast cancer risk
-
Journal of National Cancer Institute, 98 (2006), pp. 459–471
- | |
-
- Van Antwerp et al., 1996
-
Suppression of TNF-alpha-induced apoptosis by NF-kappaB
-
Science, 274 (1996), pp. 787–789
- | |
-
- Weber, 1977
-
Enzymology of cancer cells (first of two parts)
-
New England Journal of Medicine, 296 (1977), pp. 486–492
- |
-
- Wu et al., 1996
-
Inhibition of NF-kappaB/Rel induces apoptosis of murine B cells
-
EMBO Journal, 15 (1996), pp. 4682–4690
- |
-
- Zhang et al., 2004
-
Soy and isoflavone intake are associated with reduced risk of ovarian cancer in southeast china
-
Nutrition and Cancer, 49 (2004), pp. 125–130
- | |
Copyright © 2012 Committee on Chinese Medicine and Pharmacy. Production and hosting by Elsevier B.V.
Gerelateerde artikelen
- Genisteine aglycone vermindert klachten van endometriose en kan voorkomen dat endometriose uitgroeit tot een kwaadaardige vorm van kanker, bv. eierstokkanker
- Groenten en fruit verkleint risico op endometriose met ca. 40 procent en eten van rood vlees verhoogt sterk het risico op deze pijnlijke aandoening
- Preventie: Dagelijks gebruik van soja eiwitten verkleint het risico op endometrial kanker - baarmoederkanker significant met tot 33 procent voor soja eiwittengroep.





Plaats een reactie ...
Reageer op "Genisteine aglycone vermindert klachten van endometriose en kan voorkomen dat endometriose uitgroeit tot een kwaadaardige vorm van kanker, bv. eierstokkanker"