Uit de definitieve resultaten van de fase III CARES-310 studie geeft immuuntherapie met de combinatiebehandeling van Camrelizumab plus Rivoceranib (Apatinib) een statistisch significant betere ziektevrije overleving en overall overleving in vergelijking met Sorafenib bij patiënten met inoperabele gevorderde primaire leverkanker (HCC). Op basis van deze resultaten zal de combinatiebehandeling van Camrelizumab plus Rivoceranib (Apatinib) eerstelijns behandeling wroden voor deze groep van patiënten stellen de onderzoekers.
De definitieve resultaten van de fase III CARES-310 studie bevestigt eerdere gepubliceeerde resultaten dat Camrelizumab plus Rivoceranib (Apatinib) een duurzaam langere overleving laat zien ten opzichte van sorafenib als eerstelijnsbehandeling voor niet-operabele HCC - primaire leverkanker:
Camrelizumab plus Rivoceranib (Apatinib):
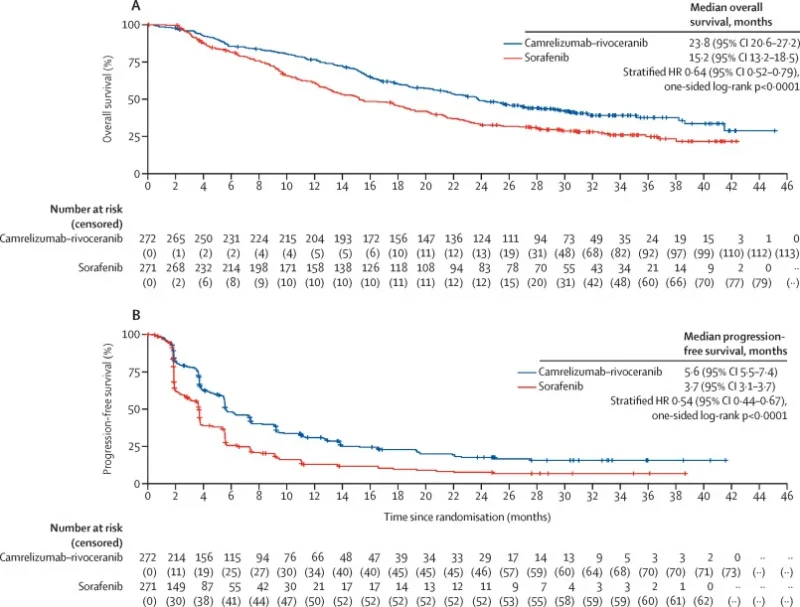
- Geeft een langere algehele overleving van 23,8 maanden versus 15,2 maanden.
- Geeft een verbeterde ziektevrije overleving plus betere respons en responsduur.
- Geeft ook een beheersbaar verwacht veiligheidsprofiel, zonder nieuwe extra ernstige bijwerkingen.
- En geeft over het algemeen behoud van kwaliteit van leven gedurende de verlengde behandeling.
Hier een grafische afbeelding van de uiteindelijke studiereultaten gekopieerd uit het studieverslag (tekst loopt verder onder afbeelding):
Bijwerkingen:
Behandelingsgerelateerde bijwerkingen kwamen in beide groepen vaak voor, met meer graad 3-4 bijwerkingen in de combinatiegroep van Camrelizumab plus Rivoceranib (Apatinib).
Graad ≥3 behandelingsgerelateerde bijwerkingen: 81% met Camrelizumab plus Rivoceranib (Apatinib) versus 54% met Sorafenib.
Immuungerelateerde bijwerkingen traden op bij 57% van de patiënten die Camrelizumab plus Rivoceranib (Apatinib) kregen (17% had graad ≥3), verder voornamelijk afwijkingen in laboratoriumwaarden en de levergalwegen, en waren over het algemeen goed te behandelen met steroïden en aanpassing van de behandeling.
De kwaliteit van leven bleef over het algemeen stabiel in de loop van de tijd, zonder grote verschillen tussen de groepen ondanks de langere behandelingsduur in de combinatiegroep met Camrelizumab plus Rivoceranib (Apatinib).
Het volledige studierapport is tegen bepaaldce voorwaarden gratis in te zien. Hier het abstract:
Summary
Background
The phase 3 CARES-310 trial showed significant improvements in progression-free survival (primary analysis) and overall survival (interim analysis) with the anti-PD-1 antibody camrelizumab plus the oral vascular endothelial growth factor receptor 2 inhibitor rivoceranib versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Here, we present the final analysis of overall survival, and updated data on progression-free survival, secondary efficacy endpoints, and safety.
Methods
This randomised, open-label, international phase 3 trial (CARES-310) was done at 95 study sites across 13 countries and regions. Eligible patients were aged 18 years or older, with unresectable or metastatic hepatocellular carcinoma, no previous systemic treatment, and an Eastern Cooperative Oncology Group performance status of 0 or 1. Participants were randomly assigned (1:1) using a centralised interactive response system to receive either camrelizumab 200 mg intravenously every 2 weeks plus rivoceranib 250 mg orally once daily or sorafenib 400 mg orally twice daily. The primary endpoints were progression-free survival, as assessed by the blinded independent review committee per Response Evaluation Criteria in Solid Tumours version 1.1, and overall survival, assessed in the intention-to-treat population. Safety was assessed in all patients who received at least one dose of the study drugs. The study is complete and was registered with ClinicalTrials.gov, NCT03764293.
Findings
Between June 28, 2019, and March 24, 2021, 543 patients (457 [84%] males; 450 [83%] were Asian) were randomly assigned to receive camrelizumab–rivoceranib (n=272) or sorafenib (n=271). At final analysis on June 14, 2023, the median follow-up was 22·1 months (IQR 11·9–30·3) in the camrelizumab–rivoceranib group and 14·9 months (7·2–28·3) in the sorafenib group. Median overall survival was 23·8 months (95% CI 20·6–27·2) with camrelizumab–rivoceranib and 15·2 months (13·2–18·5) with sorafenib (hazard ratio 0·64 [95% CI 0·52–0·79]; one-sided p<0·0001). Median progression-free survival was 5·6 months (95% CI 5·5–7·4) with camrelizumab–rivoceranib and 3·7 months (3·1–3·7) with sorafenib (HR 0·54 [0·44–0·67]; one-sided p<0·0001). The most common grade 3 or 4 treatment-related adverse events were hypertension (104 [38%] of 272 patients in the camrelizumab–rivoceranib group vs 40 [15%] of 269 patients in the sorafenib group), palmar-plantar erythrodysaesthesia syndrome (33 [12%] vs 42 [16%]), increased aspartate aminotransferase (47 [17%] vs 14 [5%]), and increased alanine aminotransferase (38 [14%] vs eight [3%]). Treatment-related serious adverse events were reported in 69 (25%) of 272 patients in the camrelizumab–rivoceranib group and 18 (7%) of 269 patients in the sorafenib group. Treatment-related deaths occurred in one patient each in the camrelizumab–rivoceranib group (multiple organ dysfunction syndrome) and sorafenib group (respiratory failure and circulatory collapse).
Interpretation
At final analysis, camrelizumab plus rivoceranib continued to show clinically meaningful survival improvement compared with sorafenib, with manageable safety. The extended follow-up further confirmed the benefit-to-risk profile of camrelizumab plus rivoceranib, supporting the combination as a new first-line treatment option for unresectable hepatocellular carcinoma.
Funding
Jiangsu Hengrui Pharmaceuticals and Elevar Therapeutics.
References
1.
Sung, H ∙ Ferlay, J ∙ Siegel, RL ∙ et al.
Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries
CA Cancer J Clin. 2021; 71:209-249
2.
Singal, AG ∙ Lampertico, P ∙ Nahon, P
Epidemiology and surveillance for hepatocellular carcinoma: New trends
J Hepatol. 2020; 72:250-261
3.
Llovet, JM ∙ Ricci, S ∙ Mazzaferro, V ∙ et al.
Sorafenib in advanced hepatocellular carcinoma
N Engl J Med. 2008; 359:378-390
4.
Cheng, AL ∙ Kang, YK ∙ Chen, Z ∙ et al.
Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial
Lancet Oncol. 2009; 10:25-34
5.
Kudo, M ∙ Finn, RS ∙ Qin, S ∙ et al.
Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial
Lancet. 2018; 391:1163-1173
6.
Finn, RS ∙ Qin, S ∙ Ikeda, M ∙ et al., the IMbrave150 Investigators
Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma
N Engl J Med. 2020; 382:1894-1905
7.
Cheng, AL ∙ Qin, S ∙ Ikeda, M ∙ et al.
Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma
J Hepatol. 2022; 76:862-873
8.
Abou-Alfa, GK ∙ Lau, G ∙ Kudo, M ∙ et al.
Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma
NEJM Evid. 2022; 1, EVIDoa2100070
9.
Rimassa, L ∙ Chan, SL ∙ Sangro, B ∙ et al.
Five-year overall survival update from the HIMALAYA study of tremelimumab plus durvalumab in unresectable HCC
J Hepatol. 2025; 83:899-908
10.
Yau, T ∙ Galle, PR ∙ Decaens, T ∙ et al., the CheckMate 9DW investigators
Nivolumab plus ipilimumab versus lenvatinib or sorafenib as first-line treatment for unresectable hepatocellular carcinoma (CheckMate 9DW): an open-label, randomised, phase 3 trial
Lancet. 2025; 405:1851-1864
11.
Liu, K ∙ Tan, S ∙ Jin, W ∙ et al.
N-glycosylation of PD-1 promotes binding of camrelizumab
EMBO Rep. 2020; 21, e51444
12.
Markham, A ∙ Keam, SJ
Camrelizumab: first global approval
Drugs. 2019; 79:1355-1361
13.
Scott, LJ
Apatinib: a review in advanced gastric cancer and other advanced cancers
Drugs. 2018; 78:747-758
14.
Qin, S ∙ Li, Q ∙ Gu, S ∙ et al.
Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial
Lancet Gastroenterol Hepatol. 2021; 6:559-568
15.
Qin, S ∙ Ren, Z ∙ Meng, Z ∙ et al.
Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial
Lancet Oncol. 2020; 21:571-580
16.
Qin, S ∙ Chan, SL ∙ Gu, S ∙ et al.
Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study
Lancet. 2023; 402:1133-1146
17.
Kelley, RK ∙ Rimassa, L ∙ Cheng, AL ∙ et al.
Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial
Lancet Oncol. 2022; 23:995-1008
18.
Llovet, JM ∙ Kudo, M ∙ Merle, P ∙ et al., the LEAP-002 Investigators
Lenvatinib plus pembrolizumab versus lenvatinib plus placebo for advanced hepatocellular carcinoma (LEAP-002): a randomised, double-blind, phase 3 trial
Lancet Oncol. 2023; 24:1399-1410
19.
Yau, T ∙ Park, JW ∙ Finn, RS ∙ et al.
Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial
Lancet Oncol. 2022; 23:77-90
20.
Qin, S ∙ Kudo, M ∙ Meyer, T ∙ et al.
Tislelizumab vs sorafenib as first-line treatment for unresectable hepatocellular carcinoma: a phase 3 randomized clinical trial
JAMA Oncol. 2023; 9:1651-1659
21.
Ghany, MG ∙ Morgan, TR, AASLD-IDSA Hepatitis C Guidance Panel
Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection
Hepatology. 2020; 71:686-721
22.
Loomba, R ∙ Liang, TJ
Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions
Gastroenterology. 2017; 152:1297-1309
23.
Tovoli, F ∙ Ielasi, L ∙ Casadei-Gardini, A ∙ et al.
Management of adverse events with tailored sorafenib dosing prolongs survival of hepatocellular carcinoma patients
J Hepatol. 2019; 71:1175-1183
24.
Raoul, JL ∙ Adhoute, X ∙ Penaranda, G ∙ et al.
Sorafenib: experience and better management of side effects improve overall survival in hepatocellular carcinoma patients: a real-life retrospective analysis
Liver Cancer. 2019; 8:457-467
25.
Xu, J ∙ Shen, J ∙ Gu, S ∙ et al.
Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial
Clin Cancer Res. 2021; 27:1003-1011
26.
Fulgenzi, CAM ∙ Scheiner, B ∙ Korolewicz, J ∙ et al.
Efficacy and safety of frontline systemic therapy for advanced HCC: a network meta-analysis of landmark phase III trials
JHEP Rep Innov Hepatol. 2023; 5, 100702
27.
Singh, H ∙ Gong, Y ∙ Roy, P ∙ et al.
FDA analysis of ECOG performance status and safety outcomes
Proc Am Soc Clin Oncol. 2020; 38, 12024
28.
Yau, T ∙ Kaseb, A ∙ Cheng, AL ∙ et al.
Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): final results of a randomised phase 3 study
Lancet Gastroenterol Hepatol. 2024; 9:310-322
29.
Johnson, PJ ∙ Berhane, S ∙ Kagebayashi, C ∙ et al.
Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade
J Clin Oncol. 2015; 33:550-558
30.
Vogel, A ∙ Cheng, A-L ∙ Shi, W ∙ et al.
Impact of baseline liver function on survival outcomes in patients with unresectable hepatocellular carcinoma (uHCC) treated with camrelizumab+ rivoceranib vs sorafenib: a post hoc analysis of study CARES-310
Proc Am Soc Clin Oncol. 2024; 42:509
(abstr).
Gerelateerde artikelen
- Camrelizumab plus Rivoceranib geeft beduidend betere overall overleving in vergelijking met Sorafenib bij patienten met inoperabele primaire leverkanker
- Immuuntherapie met Atezolizumab plus Bevacizumab - Avastin geeft betere resultaten in vergelijking met sorafenib bij inoperabele primaire leverkanker.
- Immuuntherapie: voedingspatroon, vet eten en alcoholgebruik beinvloed sterk het resultaat van immuuntherapie met interferon en ribavirin bij levertumoren (HCV) vanuit hepatitus C ontstaan.
- Immuuntherapie met anti-PD medicijnen en CAR-T-cel therapie bij primaire leverkanker geeft veelbelovende resultaten. Een reviewstudie over de stand van zaken bij immuuntherapie voor HCC



Plaats een reactie ...
Reageer op "Camrelizumab plus Rivoceranib geeft beduidend betere overall overleving in vergelijking met Sorafenib bij patienten met inoperabele primaire leverkanker"