Abstract
Purpose
Cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) have improved patient survival in hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2−) metastatic breast cancer (mBC) in clinical trials and real-world studies. However, investigations of survival gains in broader HR+/HER2− mBC populations using epidemiological approaches are limited.
Methods
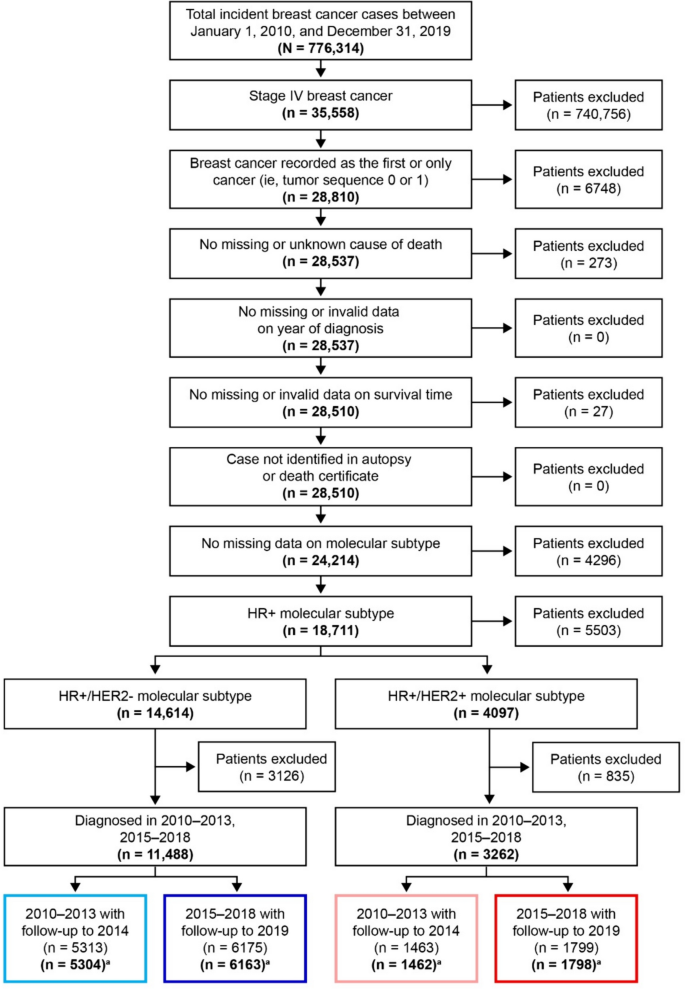
This retrospective study used SEER registry data to assess breast cancer-specific survival (BCSS) in patients diagnosed with HR+/HER2− de novo mBC from 2010 to 2019. Kaplan–Meier and Cox proportional hazards models were used to compare BCSS in patients diagnosed before (2010‒2013 with follow-up to 2014) and after (2015‒2018 with follow-up to 2019) the 2015 guideline recommendations for CDK4/6i use. A comparison was made to patients with HR+/HER2-positive (HER2+) de novo mBC, for which no major guideline changes occurred during 2015–2018.
Results
Data from 11,467 women with HR+/HER2− mBC and 3260 women with HR+/HER2+ mBC were included. After baseline characteristic adjustment, patients with HR+/HER2− mBC diagnosed post-2015 (n = 6163), had an approximately 10% reduction in risk of BC-specific death compared with patients diagnosed pre-2015 (n = 5304; HR = 0.895, p < 0.0001). Conversely, no significant change was observed in HR+/HER2+ BCSS post-2015 (n = 1798) versus pre-2015 (n = 1462). Similar results were found in patients aged ≥ 65 years.
Conclusion
Using one of the largest US population-based longitudinal cancer databases, significant improvements in BCSS were noted in patients with HR+/HER2− mBC post-2015 versus pre-2015, potentially due to the introduction of CDK4/6i post-2015. No significant improvement in BCSS was observed in patients with HR+/HER2+ mBC post-2015 versus pre-2015, likely due to the availability of HER2-directed therapies in both time periods.
Data availability
The data that support the findings of this study are available from the National Cancer Institute and can be accessed with permission under a data use agreement.
References
-
SEER Explorer (2020) Surveillance Research Program, National Cancer Institute; 2023 Apr 19. Available from: https://seer.cancer.gov/statistics-network/explorer. Accessed Sep 2023
-
SEER (2020) Cancer stat facts: Female breast cancer subtypes. Available from: https://seer.cancer.gov/statfacts/html/breast-subtypes.html. Accessed Sep 2023
-
Howlader N, Cronin KA, Kurian AW, Andridge R (2018) Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol Biomark Prev 27:619–626. https://doi.org/10.1158/1055-9965.EPI-17-0627
-
Grinda T, Antoine A, Jacot W, Blaye C, Cottu PH, Dieras V, Dalenc F, Goncalves A, Debled M, Patsouris A, Mouret-Reynier MA, Mailliez A, Clatot F, Levy C, Ferrero JM, Desmoulins I, Uwer L, Petit T, Jouannaud C, Lacroix-Triki M, Deluche E, Robain M, Courtinard C, Bachelot T, Brain E, Perol D, Delaloge S (2021) Evolution of overall survival and receipt of new therapies by subtype among 20 446 metastatic breast cancer patients in the 2008–2017 ESME cohort. ESMO Open 6:100114. https://doi.org/10.1016/j.esmoop.2021.100114
-
Iwase T, Shrimanker TV, Rodriguez-Bautista R, Sahin O, James A, Wu J, Shen Y, Ueno NT (2021) Changes in overall survival over time for patients with de novo metastatic breast cancer. Cancers (Basel). https://doi.org/10.3390/cancers13112650
-
Sundquist M, Brudin L, Tejler G (2017) Improved survival in metastatic breast cancer 1985–2016. Breast 31:46–50. https://doi.org/10.1016/j.breast.2016.10.005
-
US Food & Drug Administration (2015) Palbociclib (IBRANCE). https://www.fda.gov/drugs/resources-information-approved-drugs/palbociclib-ibrance
-
US Food & Drug Administration (2017) Ribociclib (Kisqali). https://www.fda.gov/drugs/resources-information-approved-drugs/ribociclib-kisqali
-
US Food & Drug Administration (2018) FDA approves new treatment (Verzenio, abemaciclib) for certain advanced or metastatic breast cancers. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-certain-advanced-or-metastatic-breast-cancers
-
Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, Zirkelbach JF, Yu J, Liu Q, Zhao L, Crich J, Chen XH, Hughes M, Bloomquist E, Tang S, Sridhara R, Kluetz PG, Kim G, Ibrahim A, Pazdur R, Cortazar P (2015) FDA approval: palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res 21:4760–4766. https://doi.org/10.1158/1078-0432.ccr-15-1185
-
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Zhang K, Theall KP, Jiang Y, Bartlett CH, Koehler M, Slamon D (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17:425–439. https://doi.org/10.1016/S1470-2045(15)00613-0
-
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Dieras V, Slamon DJ (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375:1925–1936. https://doi.org/10.1056/NEJMoa1607303
-
Gao JJ, Cheng J, Bloomquist E, Sanchez J, Wedam SB, Singh H, Amiri-Kordestani L, Ibrahim A, Sridhara R, Goldberg KB, Theoret MR, Kluetz PG, Blumenthal GM, Pazdur R, Beaver JA, Prowell TM (2020) CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US Food and Drug Administration pooled analysis. Lancet Oncol 21:250–260. https://doi.org/10.1016/s1470-2045(19)30804-6
-
Gao JJ, Cheng J, Prowell TM, Bloomquist E, Tang S, Wedam SB, Royce M, Krol D, Osgood C, Ison G, Sridhara R, Pazdur R, Beaver JA, Amiri-Kordestani L (2021) Overall survival in patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer treated with a cyclin-dependent kinase 4/6 inhibitor plus fulvestrant: a US Food and Drug Administration pooled analysis. Lancet Oncol 22:1573–1581. https://doi.org/10.1016/S1470-2045(21)00472-1
-
Goetz MP (2023) MONARCH 3: final overall survival results of abemaciclib plus a nonsteroidal aromatase inhibitor as first-line therapy in patients with HR+, HER2− advanced breast cancer. Cancer Res Presentation at SABCS 2023. Abstract GS01-12.
-
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, Tredan O, Chen SC, Manso L, Freedman OC, Garnica Jaliffe G, Forrester T, Frenzel M, Barriga S, Smith IC, Bourayou N, Di Leo A (2017) MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35:3638–3646. https://doi.org/10.1200/JCO.2017.75.6155
-
Goyal RK, Chen H, Abughosh SM, Holmes HM, Candrilli SD, Johnson ML (2023) Overall survival associated with CDK4/6 inhibitors in patients with HR+/HER2- metastatic breast cancer in the United States: A SEER-Medicare population-based study. Cancer 129:1051–1063. https://doi.org/10.1002/cncr.34675
-
Harbeck N, Bartlett M, Spurden D, Hooper B, Zhan L, Rosta E, Cameron C, Mitra D, Zhou A (2021) CDK4/6 inhibitors in HR+/HER2- advanced/metastatic breast cancer: a systematic literature review of real-world evidence studies. Future Oncol 17:2107–2122. https://doi.org/10.2217/fon-2020-1264
-
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L, Campone M, Petrakova K, Winer EP, Janni W, Conte P, Cameron DA, Andre F, Arteaga CL, Zarate JP, Chakravartty A, Taran T, Le Gac F, Serra P, O’Shaughnessy J (2022) Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med 386:942–950. https://doi.org/10.1056/NEJMoa2114663
-
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, Campone M, Blackwell KL, Andre F, Winer EP, Janni W, Verma S, Conte P, Arteaga CL, Cameron DA, Petrakova K, Hart LL, Villanueva C, Chan A, Jakobsen E, Nusch A, Burdaeva O, Grischke EM, Alba E, Wist E, Marschner N, Favret AM, Yardley D, Bachelot T, Tseng LM, Blau S, Xuan F, Souami F, Miller M, Germa C, Hirawat S, O’Shaughnessy J (2016) Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 375:1738–1748. https://doi.org/10.1056/NEJMoa1609709
-
Llombart-Cussac A, Sledge GW, Masakazu T, Neven P, Sohn JH, Inoue K, Pivot X, Okera M, Masuda N, Kaufman PA, Koh H, Grischke EM, Conte P, Andre V, Bian Y, Shahir A, van Hal G (2023) PD13–11 Final overall survival analysis of Monarch 2: A phase 3 trial of abemaciclib plus fulvestrant in patients with hormone receptor-positive, HER2-negative advanced breast cancer. Cancer Res 83:13–11. https://doi.org/10.1158/1538-7445.SABCS22-PD13-11
-
Rugo HS, Brufsky A, Liu X, Li B, McRoy L, Chen C, Layman RM, Cristofanilli M, Torres MA, Curigliano G, Finn RS, DeMichele A (2022) Real-world study of overall survival with palbociclib plus aromatase inhibitor in HR+/HER2- metastatic breast cancer. NPJ Breast Cancer 8:114. https://doi.org/10.1038/s41523-022-00479-x
-
Slamon D, Dieras V, Rugo HS, Harbeck N, Im SA, Gelmon K, Lipatov O, Walshe JM, Martin M, Mac Gregor MC, Bananis E, Gauthier E, Lu DR, Kim S, Finn RS (2023) Overall survival with palbociclib plus letrozole in advanced breast cancer. J Clin Oncol. https://doi.org/10.1200/JCO.23.00137
-
Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, Andre F, Barrios CH, Bergh J, Bhattacharyya GS, Biganzoli L, Boyle F, Cardoso MJ, Carey LA, Cortes J, El Saghir NS, Elzayat M, Eniu A, Fallowfield L, Francis PA, Gelmon K, Gligorov J, Haidinger R, Harbeck N, Hu X, Kaufman B, Kaur R, Kiely BE, Kim SB, Lin NU, Mertz SA, Neciosup S, Offersen BV, Ohno S, Pagani O, Prat A, Penault-Llorca F, Rugo HS, Sledge GW, Thomssen C, Vorobiof DA, Wiseman T, Xu B, Norton L, Costa A, Winer EP (2020) 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 31:1623–1649. https://doi.org/10.1016/j.annonc.2020.09.010
-
McAndrew NP, Finn RS (2022) Clinical review on the management of hormone receptor-positive metastatic breast cancer. JCO Oncol Pract 18:319–327. https://doi.org/10.1200/OP.21.00384
-
Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, Fallowfield L, Fowble B, Ingle JN, Jahanzeb M, Johnston SR, Korde LA, Khatcheressian JL, Mehta RS, Muss HB, Burstein HJ (2016) Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology guideline. J Clin Oncol 34:3069–3103. https://doi.org/10.1200/JCO.2016.67.1487
-
Waks AG, Winer EP (2019) Breast cancer treatment: a review. JAMA 321:288–300. https://doi.org/10.1001/jama.2018.19323
-
Alvarez A, Bernal AM, Anampa J (2023) Racial disparities in overall survival after the introduction of cyclin-dependent kinase 4/6 inhibitors for patients with hormone receptor-positive, HER2-negative metastatic breast cancer. Breast Cancer Res Treat 198:75–88. https://doi.org/10.1007/s10549-022-06847-2
-
DCCPS. SRP (2020) National Cancer Institute. Bethesda, MD. Available from: https://seer.cancer.gov/registries/terms.html.
-
Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA (2010) Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst 102:1584–1598. https://doi.org/10.1093/jnci/djq366
-
Chang JC, O’Regan R (2018) Use of novel combination therapies in the treatment of advanced HR+/HER2− breast cancer. J Natl Compr Canc Netw 16:S5–S17. https://doi.org/10.6004/jnccn.2018.0200
-
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, Petrakova K, Bianchi GV, Esteva FJ, Martin M, Nusch A, Sonke GS, De la Cruz-Merino L, Beck JT, Pivot X, Vidam G, Wang Y, Rodriguez Lorenc K, Miller M, Taran T, Jerusalem G (2018) Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 36:2465–2472. https://doi.org/10.1200/JCO.2018.78.9909
-
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, Koh H, Grischke EM, Frenzel M, Lin Y, Barriga S, Smith IC, Bourayou N, Llombart-Cussac A (2017) MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35:2875–2884. https://doi.org/10.1200/JCO.2017.73.7585
-
Llombart-Cussac A, Perez-Garcia JM, Bellet M, Dalenc F, Gil-Gil M, Ruiz-Borrego M, Gavila J, Schmid P, Zamora P, Wheatley D, Martinez-de Deunas E, Amillano K, Anton A, Cottu P, Vinas G, Petit T, Tesarova P, Ceuva J, Colleoni M, Martinez del Prado MP, Andres R, Aguirre E, Diaz M, Vitorino S, Sampayo-Cordero M, Cortes J (2023) PARSIFAL-LONG: Extended follow-up of hormone receptorpositive/HER2-negative advanced breast cancer patients treated with fulvestrant and palbociclib vs letrozole and palbociclib in the PARSIFAL study. Cancer Res Presentation at SABCS 2023.
-
Cottu P, Ramsey SD, Sola-Morales O, Spears PA, Taylor L (2022) The emerging role of real-world data in advanced breast cancer therapy: Recommendations for collaborative decision-making. Breast 61:118–122. https://doi.org/10.1016/j.breast.2021.12.015
-
Swain SM, Miles D, Kim SB, Im YH, Im SA, Semiglazov V, Ciruelos E, Schneeweiss A, Loi S, Monturus E, Clark E, Knott A, Restuccia E, Benyunes MC, Cortes J, group Cs (2020) Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 21:519–530. https://doi.org/10.1016/S1470-2045(19)30863-0
-
Ibragimova KIE, Geurts SME, Croes S, Erdkamp F, Heijns JB, Tol J, Vriens B, Aaldering KNA, Dercksen MW, Pepels M, Peters N, van de Winkel L, Tilli DJP, Vriens IJH, de Boer M, Tjan-Heijnen VCG (2021) Survival before and after the introduction of pertuzumab and T-DM1 in HER2-positive advanced breast cancer, a study of the SONABRE Registry. Breast Cancer Res Treat 188:571–581. https://doi.org/10.1007/s10549-021-06178-8
-
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A, Jemal A, Siegel RL (2022) Breast cancer statistics, 2022. CA Cancer J Clin 72:524–541. https://doi.org/10.3322/caac.21754
-
Singh H, Kanapuru B, Smith C, Fashoyin-Aje LA, Myers A, Kim G, Pazdur R (2017) FDA analysis of enrollment of older adults in clinical trials for cancer drug registration: A 10-year experience by the US Food and Drug Administration. J Clin Oncol 35:10009. https://doi.org/10.1200/JCO.2017.35.15_suppl.10009
-
Talarico L, Chen G, Pazdur R (2004) Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol 22:4626–4631. https://doi.org/10.1200/JCO.2004.02.175
-
Cherny NI, Sullivan R, Dafni U, Kerst JM, Sobrero A, Zielinski C, de Vries EG, Piccart MJ (2015) A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol 26:1547–1573. https://doi.org/10.1093/annonc/mdv249
-
Ellis LM, Bernstein DS, Voest EE, Berlin JD, Sargent D, Cortazar P, Garrett-Mayer E, Herbst RS, Lilenbaum RC, Sima C, Venook AP, Gonen M, Schilsky RL, Meropol NJ, Schnipper LE (2014) American Society of Clinical Oncology perspective: raising the bar for clinical trials by defining clinically meaningful outcomes. J Clin Oncol 32:1277–1280. https://doi.org/10.1200/JCO.2013.53.8009
-
Jaber Chehayeb R, Hood A, Wang X, Miksad R, Schellhorn Mougalian S, Lustberg MB, Wang SY, Greenup RA, Pusztai L, Kunst N (2022) Treatment sequencing patterns and associated direct medical costs of metastatic breast cancer care in the United States, 2011 to 2021. JAMA Netw Open 5:e2244204. https://doi.org/10.1001/jamanetworkopen.2022.44204
-
Goldschmidt D, Dalal AA, Romdhani H, Kelkar S, Guerin A, Gauthier G, Wu EQ, Niravath P, Small T (2018) Current treatment patterns among postmenopausal women with HR+/HER2− metastatic breast cancer in US community oncology practices: an observational study. Adv Ther 35:482–493. https://doi.org/10.1007/s12325-018-0676-2
-
Xi J, Oza A, Thomas S, Ademuyiwa F, Weilbaecher K, Suresh R, Bose R, Cherian M, Hernandez-Aya L, Frith A, Peterson L, Luo J, Krishnamurthy J, Ma CX (2019) Retrospective analysis of treatment patterns and effectiveness of palbociclib and subsequent regimens in metastatic breast cancer. J Natl Compr Canc Netw 17:141–147. https://doi.org/10.6004/jnccn.2018.7094
-
Ha MJ, Singareeka Raghavendra A, Kettner NM, Qiao W, Damodaran S, Layman RM, Hunt KK, Shen Y, Tripathy D, Keyomarsi K (2022) Palbociclib plus endocrine therapy significantly enhances overall survival of HR+/HER2- metastatic breast cancer patients compared to endocrine therapy alone in the second-line setting: a large institutional study. Int J Cancer 150:2025–2037. https://doi.org/10.1002/ijc.33959
-
Liu Y, Wu J, Ji Z, Chen L, Zou J, Zheng J, Lin W, Cai J, Chen Y, Zheng D, Chen Y, Li Z (2023) Comparative efficacy and safety of different combinations of three CDK4/6 inhibitors with endocrine therapies in HR+/HER-2 - metastatic or advanced breast cancer patients: a network meta-analysis. BMC Cancer 23:816. https://doi.org/10.1186/s12885-023-11322-2
-
SEER (2020) SEER registries: Number of persons by race and Hispanic ethnicity for SEER participants (2020 census data). Available from: https://seer.cancer.gov/registries/data.html. Accessed Feb 2024
Funding
This work was supported by Pfizer Inc. Medical writing support was provided by Kevin Woolfrey, PhD, of Oxford PharmaGenesis Inc., (Newtown, PA, USA) and was funded by Pfizer Inc.
Author information
Authors and Affiliations
Contributions
All authors assisted with the development, review and approval of the submitted manuscript.
Corresponding author
Ethics declarations
Competing interest
AB reports consultancy fees from AstraZeneca, Pfizer, Novartis, Lilly, Genentech/Roche, Seagen, Daiichi-Sankyo, Merck, Agendia, Sanofi, and Puma, and provides research support to Agendia and AstraZeneca MLK reports ongoing collaboration on a Pfizer-funded study. SS, RS, AC, DM are employees and stockholders of Pfizer Inc. SK and RKG are full-time employees of RTI Health Solutions, an independent nonprofit research organization, which was a paid consultant to Pfizer in connection with the development of this manuscript. Their compensation is unconnected to the studies on which they work.
Consent to participate
Patient consent was not required for this anonymized, non-interventional study.
Ethical approval
The study was reviewed by the RTI International Institutional Review Board and received a determination of “not research involving human subjects.”
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.



Plaats een reactie ...
Reageer op "CDK 4/6-remmers (CDK4/6i) blijken overall overleving van patienten met borstkanker HRplus/HER2 negatief te verbeteren."