17 maart 2021: Aanvullend op onderstaande informatie hier het volledige studierapport Low-dose cyclophosphamide selectively expands resident anti-tumor Tcells allowing in situ control of colorectal cancer in PDF formaat.
28 maart 2017 lees ook dit artikel:
https://kanker-actueel.nl/NL/immuuntherapie-geeft-uitstekende-resultaten-bij-darmkanker-met-minimale-ziekte-weinig-tumoren-en-zelfs-bij-darmkanker-stadium-iv-werkt-het-ook-al-is-het-dan-minder-effectief.html
28 maart 2017. Bron: ASCO-SITC (Society for Immunotherapy of Cancer) Clinical Immuno-Oncology Symposium
Immuuntherapie met een gemoduleerd virus, het zogeheten Ankara-ST4 virus, plus een lage dosis cyclophosphamide geeft uitstekende resultaten bij eerder met chemo voorbehandelde inoperabele darmkankerpatienten met vergevorderde darmkanker. Progressievrije ziektetijd ging van 2,4 naar 5 maanden en mediane overall overleving van 11,2 naar 20,0 maanden in de groep patiënten met de optimale behandeling.
Dit blijkt uit een kleinschalige open-label fase I/II veiligheidsstudie bij totaal 52 patienten met inoperabele darmkanker.
Bijna alle patiënten hadden eerder een chemokuur gehad met of capecitabine (Xeloda) en 5-FU (fluorouracil) en de meesten hadden daarna ook nog andere chemokuren of behandelingen gehad.
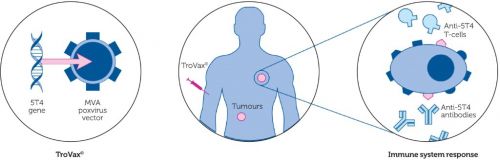 Werkingsmechanisme van het Ankara virus (TroVax)
Werkingsmechanisme van het Ankara virus (TroVax)
Studie opzet:
Patiënten werden gerandomiseerd ingedeeld in geen behandeling (n = 8), of een lage dosis cyclophosphamide met 50 mg 2x daags gedurende behandelingsweken 1 en 3 (n = 9), of een vaccin met het gemoduleerde virus Ankara–5T4 only (n = 17), of lage dosis cyclophosphamide gevolgd door het gemoduleerde virus Ankara–5T4 (n = 18). Patiënten in de vaccingroep startten de behandeling op dag 22, nadat zij eerder cyclophosphamide hadden gekregen. Zij kregen 5 injecties om de 2 weken de 6e injectie 4 weken na de 5e injectie. Het primaire doel was te zien of de behandeling een boost te zien gaf in de anti-5T4 genen op dag 43 door het meten van gestegen T-cellen en/of antibody responses.
Kernpunten uit de studie:
- A novel therapeutic vaccine employs a highly attenuated strain of vaccinia virus, modified vaccinia virus Ankara, and encodes the tumor antigen 5T4, which is found on 90% of colorectal cancers.
- In a phase I/II trial of advanced colorectal cancer patients, modified vaccinia virus Ankara–5T4 plus low-dose cyclophosphamide (delivered prior to vaccination) led to robust immune responses that were associated with improved progression-free and overall survival.
- Low-dose cyclophosphamide alone also produced strong immune responses that were associated with prolonged remission.
Een opmerkelijk citaat van de studieleider:
This is the first randomized study to show a clear benefit of immunotherapy in advanced colorectal cancer—and to suggest this approach may be superior to (and less toxic than) continuous palliative chemotherapy in these patients.
— Martin Scurr, PhD
Studie resultaten:
“Nadat we de cyclofosfamide gaven, zagen we dat de patiënten met de grootste vermindering van regulerende onderdrukkende T-cellen het meeste profiteerden met een progressievrije overleving,” aldus Dr. Scurr gemeld. “Gewoon het geven van een lage dosis cyclofosfamide alleen gaf ook al een immuunrespons te zien.”
Cyclophosphamide verzwakte regulerende onderdrukkende T cellen bij 21 van de 27 patiënten gedurende de eerste drie weken (P = .0028). Onder de 27 met cyclophosphamide behandelde patiënten, bereikten er 12 een vermindering van minimaal 39.4% (het doel dat statistisch significantie betekende). Deze verzwakking van de regulerende T-cellen werd ook gerelateerd aan een langere progressie vrije ziekte in de groepen die ook cyclophosphamide hadden gekregen, vergeleken met de groep patiënten die niet de 39,4% vermindering hadden gehaald. Mediane progressie vrije ziekte werd 5.0 vs 2.4 maanden voor deze groep van patiënten. (hazard ratio = 0.48, P = .09).
Een vaccinatie erbij verdubbelde de anti-5T4 immuun reacties bij 16 van de 35 patiënten welke waren behandeld met het gemoduleerde virus Ankara–5T4. Deze patiënten ervaarden een progressievrije ziekte van 5,6 maanden versus 2,4 maanden. (HR = 0.21, P = .0002) en een mediane overall overleving van 20.0 maanden vs 11.2 maanden; HR = 0.32, P = .0076).
Belangrijk te melden dat er geen extra ernstige bijwerkingen optraden bij deze aanpak.
In dit studie rapport, vrij in te zien: TroVax in colorectal cancer wordt beschreven hoe TROVAX werkt en gaat vergezeld van een interessante referentielijst. Zie ook hieronder. Interessant is ook dat Trovax bij ook andere vormen van kanker b.v. nierkanker, prostaatkanker wordt onderzocht en hoopvolle resultaten geeft.
Een andere studie van immuuntherapie met het gemoduleerde virus Ankara is deze review studie: 5T4-modified vaccinia Ankara: progress in tumor-associated antigen-based immunotherapy maar daarvoor moet worden betaald. En het abstract geeft te weinig informatie om daaruit conclusies op te kunnen maken.
Het studierapport: Scurr M, Pembroke T, Adams R et al: MVA-5T4 immunotherapy and low-dose cyclophosphamide for advanced colorectal cancer (TaCTiCC): An open-label, randomized phase I/II trial is nog niet vrij in te zien.
Hieronder wel het abstract na het abstract en referentielijst van de eerder genoemde studie: TroVax in colorectal cancer
The existing results support the ability of TroVax to induce immune responses within the host;
TroVax in colorectal cancer
Abstract
Currently, the backbone of therapy for metastatic disease is cytotoxic chemotherapy, along with the recent addition of targeted therapy based on molecular markers with KRAS testing. Despite the improvement in survival for metastatic colon cancer, newer agents are still needed. The clinical activity of TroVax in metastatic colon cancer has been studied in a small number of clinical trials. There is evidence that supports the vaccine's ability to induce humoral and cellular responses, as demonstrated by positive 5T4 and MVA-specific antibody titers and cellular proliferation assays. Future strategies should focus on investigating the immunomodulatory effects of chemotherapy in conjunction with TroVax, understanding the optimal dosing and schedule of the combination, and examining potential predictive biomarkers to determine which patients may benefit from immunotherapy from those who do not.
Abbreviations
- ADCC
- Antibody-dependent cell-mediated cytotoxicity
- CEA
- Carcinoembryonic antigen
- CRC
- Colorectal cancer
- DT
- Doubling time
- EBNA-1
- Epstein Barr-Virus nuclear antigen-1
- EGFR
- Epidermal growth factor receptor
- HRPC
- Hormone refractory prostate cancer
- IHC
- Immunohistochemoical
- ITT
- Intention to treat
- LMP-2
- Latent membrane protein-2 antigens
- mCRC
- Metastatic colon cancer
- mRCC
- Metastatic renal cell carcinoma
- MSKCC
- Memorial Sloan-Kettering Cancer Center
- MVAs
- Modified vaccinia Ankara
- NSCLC
- Non-small cell lung cancer
- OS
- Overall survival
- PD-1
- Programmed death 1 receptor
- PD-L1
- Programmed-death ligand 1
- PFS
- Progression free survival
- PMNs
- Peripheral blood mononuclear cells
- RCC
- Renal cell carcinoma
- TAAs
- Tumor-associated antigens
- T-FOLFOX
- Trovax and FOLFOX
- T-FOLFIRI
- Trovax and FOLFIRI
- TILs
- Tumor-infiltrating lymphocytes
- TTP
- Time to progression
- VEGF
- Vascular-endothelial growth factor
-
References
2.
Kemeny NE.. Treatment of metastatic colon cancer: “the times they are A- changing.” J Clin Oncol 2013; 31:1913-6; PMID:23630214; http://dx.doi.org/10.1200/JCO.2013.49.4500 [PubMed] [Cross Ref]3.
Sourrouille I., Mordant P., Maggiori L., Dokmak S., Leseche G., Panis Y., Belghiti J., Castier Y.. Long term survival after hepatic and pulmonary resection of the colorectal cancer metastases. J Surg Oncol 2013; 108:220-4; PMID:23893480; http://dx.doi.org/10.1002/jso.23385 [PubMed] [Cross Ref]4.
Elkord E., Dangoor A., Drury NL., Harrop R., Burt DJ., Drijfhout JW., Hamer C., Andrews D., Naylor S., Sherlock D, et al. An MVA- based vaccine targeting the oncofetal antigen 5T4 in patients undergoing surgical resection of colorectal cancer liver metastases. J Immunother 2008; 31:820-9; PMID:18833005; http://dx.doi.org/10.1097/CJI.0b013e3181876ab3 [PubMed] [Cross Ref]5.
Quan D., Gallinger S., Nhan C., Auer RA., Biagi JJ., Fletcher GG., Law CH., Moulton CA., Ruo L., Wei AC., Mcleod RS.. The role of liver resection for colorectal cancer metastases in an era of multimodality treatment: a systematic review. Surgery 2012; 151:860-70; PMID:22316439; http://dx.doi.org/10.1016/j.surg.2011.12.018 [PubMed] [Cross Ref]6.
Kantoff PW., Higano CS., Shore ND., Berger ER., Small EJ., Penson DF., Redfern CH., Ferrari AC., Dreicer R., Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411-22; PMID:20818862; http://dx.doi.org/10.1056/NEJMoa1001294 [PubMed] [Cross Ref]7.
Schwartzentruber DJ., Lawson DH., Richards JM., Contry RM., Miller DM., Terisman J., Gailani F., Riley L., Conlon K., Pockaj B, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 2011; 364:2119-27; PMID:21631324; http://dx.doi.org/10.1056/NEJMoa1012863 [PMC free article] [PubMed] [Cross Ref]8.
Hodi FS., O’Day SJ., McDermott DF., Weber RW., Sosman JA., Haanen JB., Gonzalez R., Robert C., Schadendorf D., Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466 [PMC free article] [PubMed] [Cross Ref]9.
Bloch O., Crane CA., Fuks Y., Kaur R., Aghi MK., Berger MS., Butowski NA., Chang SM., Clarke JL., McDermott MW, et al. Heat-shock protein peptide complex–96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro Oncol 2014; 16:274-9. not203 202014; PMID:24335700; http://dx.doi.org/10.1093/neuonc/not203 [PMC free article] [PubMed] [Cross Ref]10.
Strbo N., Garcia-Soto A., Schreiber TH., Podack ER.. Secreted heat shock protein gp96-Ig: next-generation vaccines for cancer and infectious diseases. Immunol Res 2013; 57:311-25; PMID:24254084; http://dx.doi.org/10.1007/s12026-013-8468-x [PubMed] [Cross Ref]11.
Morris DG., Feng X., DiFrancesco LM., Fonseca K., Forsyth PA., Paterson AH., Coffey MC., Thompson B.. REO-001: a phase I trial of percutaneous intralesional administration of reovirus type 3 dearing (Reolysin) in patients with advanced solid tumors. Invest New Drugs 2013; 31:696-706; PMID:22886613; http://dx.doi.org/10.1007/s10637-012-9865-z [PubMed] [Cross Ref]12.
Dolan DE., Gupta S.. PD-1 pathway inhibitors: changing the landscape of cancer immunotherapy. Cancer Control 2014; 21:231-37; PMID:24955707 [PubMed]13.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev 2012; 12:251-64. [PMC free article] [PubMed]14.
Said R., mato RJ.. Identification of pre- and post-treatment markers, clinical, and laboratory parameters associated with outcome in renal cell cancer patients treated with MVA-5T4. Front Oncol 2013; 3:185; PMID:23875174; http://dx.doi.org/10.3389/fonc.2013.00185 [PMC free article] [PubMed] [Cross Ref]15.
Larocca C., chlom J. Viral vector-based therapeutic cancer vaccines. Cancer J 2011; 5:359-71; http://dx.doi.org/10.1097/PPO.0b013e3182325e63 [PMC free article] [PubMed] [Cross Ref]16.
Kim DW., Krishnamurthy V., Bines SB., Kaufman HL.. TroVax, a recombinant modified vaccinia Ankara virus encoding 5T4- lessons learned and future development. Hum Vaccin 2010; 6:784-91; PMID:20975327; http://dx.doi.org/10.4161/hv.6.10.13144 [PubMed] [Cross Ref]17.
Harrop R., Connolly N., Redchenko I., Valle J., Saunders M., Ryan MG., Myers KA., Drury N., Kingsman SM., Hawkins RE, et al. Vaccination of colorectal cancer patients with modified vaccinia ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease Control: a phase III trial. Clin Cancer Res 2006; 12:3416-24; PMID:16740766; http://dx.doi.org/10.1158/1078-0432.CCR-05-2732 [PubMed] [Cross Ref]18.
Harrop R., Drury N., Shingler W., Chikoti P., Redchenko I., Carroll MW., Kingsman SM., Naylor S., Melcher A., Nicholls J, et al. Vaccination of colorectal cancer patients with modified vaccinia ankara encoding the tumor antigen 5T4 (TroVax) given alongside chemotherapy induces potent immune responses. Clin Cancer Res 2007; 13:4487-94; PMID:17671134; http://dx.doi.org/10.1158/1078-0432.CCR-07-0704 [PubMed] [Cross Ref]19.
Harrop R., Noel Drury W., Shingler P., Chikoti I., Redchenko I., Carroll MW., Kingsman SM., Naylor S., Griffiths R., Steven N, et al. Vaccination of colorectal cancer patients with TroVax given alongside chemotherapy (5-fluorouracil, leukovorin an irinotecan) is safe and induced potent immune responses. Cancer Immunol Immunother 2008; 57:977-86; PMID:18060404; http://dx.doi.org/10.1007/s00262-007-0428-7 [PubMed] [Cross Ref]20.
Oudard S., Rixe O., Beuselinck B., Linassier C., Banu E., Machiels JP., Baudard M., Ringeisen F., Velu T., Lefrere-Belda MA, et al. A phase II study of the cancer vaccine TG4010 alone and in combination with cytokines in patients with metastatic renal cell-clear-cell carcinoma: clinical and immunological findings. Cancer Immuno Immunother 2011; 60:261-71; http://dx.doi.org/10.1007/s00262-010-0935-9 [PubMed] [Cross Ref]21.
Dreicer R., Stadler WM., Ahmann FR., Whiteside T., Bizouarne N., Acres B., Limacher JM., Squiban P., Pantuck A.. MVA-MUC1-IL2 vaccine immunotherapy (TG4010) improves PSA doubling time in patients with prostate cancer with biochemical failure. Invest New Drugs 2009; 27:379-86; PMID:18931824; http://dx.doi.org/10.1007/s10637-008-9187-3 [PubMed] [Cross Ref]22.
Ramlau R., Quoix E., Rolski J., Pless M., Lena H., Levy E., Krzakowski M., Hess D., Tartour E., Chenard MP, et al. A phase II study of TG4010 (MVA-MUC1-IL1) in association with chemotherapy in patients with Stage IIIIV Non-Small Cell Lung cancer. J Thorac Oncol 2008; 3:735-44; PMID:18594319; http://dx.doi.org/10.1097/JTO.0b013e31817c6b4f [PubMed] [Cross Ref]24.
National Institutes of Health Clinical Center. Safety and Tolerability of a Modified Vaccinia Ankara (MVA)-Based Vaccine Modified to Express Brachyury and T-cell Costimulatory Molecules (MVA-Brachyury-TRICOM) https:clinicaltrials.govct2showNCT02179515, July 28, 201425.
Swiderska M., Choromańska B., Dąbrowska E., Konarzewska-Duchnowska E., Choromańska K., Szczurko G., Myśliwiec P., Dadan J., Ładny JR., Zwierz K. The diagnostics of colorectal cancer. Contemp Oncol 2014; 18:1-6. [PMC free article] [PubMed]26.
Locker GY., Hamilton S., Harris J., Jessup JM., Kemeny N., Macdonald JS., Somerfield MR., Hayes DF., Bast RC., Jr ASCO 2006 Update of recommendations for the use of tumor markers in gastrointrestinal cancer. J Clin Oncol 2006; 33:5313-27. [PubMed]27.
Finn OJ.. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol 2012; 23 (supplement 8): viii6-9; PMID:22918931 [PMC free article] [PubMed]28.
Lake RA., Robinson BW.. Immunotherapy and chemotherapy–a practical partnership. Nat Rev Cancer 2005; 5:397-405; PMID:15864281 [PubMed]29.
Hawkins RE., MacDermott C., Shablak A., Hamer C., Thistlethwaite F., Drury NL., Chikoti P., Shingler W., Naylor S., Harrop R.. Vaccination of patients with metastatic renal cancer with modified vaccinia Ankara encoding the tumor antigen 5T4 (TroVax) given alongside interferon-alpha. J Immunother 2009; 32:424-9; PMID:19342962; http://dx.doi.org/10.1097/CJI.0b013e31819d297e [PubMed] [Cross Ref]30.
Amato RJ., Shingler W., Goonewardena M., de Belin J., Naylor S., Jac J., Willis J., Saxena S., Hernandez-McClain J., Harrop R.. Vaccination of renal cell cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) alone or administered with interferon-alpha (IFN-alpha): a phase 2 trial. J Immunother 2009; 32:765-72; PMID:19561532; http://dx.doi.org/10.1097/CJI.0b013e3181ace876 [PubMed] [Cross Ref]31.
Amato RJ., Shingler W., Naylor S., Jac J., Willis J., Saxena S., Hernandez-McClain J., Harrop R.. Vaccination of renal cell cancer patients with modified vaccinia Ankara delivering tumor antigen 5T4 (TroVax) administered with interleukin 2: a phase II trial. Clin Cancer Res 2008; 14:7504-10; PMID:19010868; http://dx.doi.org/10.1158/1078-0432.CCR-08-0668 [PubMed] [Cross Ref]32.
Kaufman H., Taback B., Sherman W., Kim DW., Shingler WH., Moroziewicz D., DeRaffele G., Mitcham J., Carroll MW., Harrop R, et al. Phase II trial of Modified Vaccinia Ankara (MVA) virus expressing 5T4 and high dose Interleukin-2 (IL-2) in patients with metastatic renal cell carcinoma. J Transl Med 2009; 7:2; PMID:19128501; http://dx.doi.org/10.1186/1479-5876-7-2 [PMC free article] [PubMed] [Cross Ref]33.
Amato RJ., Drury N., Naylor S., Jac J., Saxena S., Cao A., Hernandez-McClain J., Harrop R.. Vaccination of prostate cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax): a phase 2 trial. J Immunother 2008; 31:577-85; PMID:18528296; http://dx.doi.org/10.1097/CJI.0b013e31817deafd [PubMed] [Cross Ref]34.
Cadriff University. A pilot study to assess the effect of regulatory T cell depletion on 5T4-containing MVA (TROVAX®) vaccination in patients with INOPERABLE metastatic colorectal cancer (TaCTiCC) www.controlled-trials.comISRCTN54669986, April 17, 201435.
Lutsiak ME., Semnani RT., De Pascalis R., Kashmiri SV., Schlom J., Sabzevari H.. Inhibition of CD4 (+) 25 +T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005; 105:2862-8; PMID:15591121; http://dx.doi.org/10.1182/blood-2004-06-2410 [PubMed] [Cross Ref]
Conclusions: Both CPM and TroVax induced highly beneficial anti-tumor immune responses resulting in significantly prolonged survival of end-stage CRC patients without toxicity. This is the first study to show a clear benefit of immunotherapy in advanced CRC, and suggests this approach may be superior (and less toxic) to continuous palliative chemotherapy in these patients.
MVA-5T4 immunotherapy and low-dose cyclophosphamide for advanced colorectal cancer (TaCTiCC): An open-label, randomized phase I/II trial.
Category:
Therapies Targeting T cells
Session Type and Session Title:
Citation:
J Clin Oncol 35, 2017 (suppl 7S; abstract 154)
Author(s):
Martin Scurr, Tom Pembroke, Richard Adams, Daniel Blount, Alison Brewster, Sarah Gwynne, Richard Harrop, Robert Jones, Robert Hills, Awen Gallimore, Andrew Godkin; Cardiff University School of Medicine, Cardiff, United Kingdom; Velindre Cancer Centre, Cardiff, United Kingdom; Oxford Biomedica, Oxford, United Kingdom; South West Wales Cancer Centre, Swansea, United Kingdom
Background: Current immunotherapies including checkpoint inhibitors and vaccines for advanced colorectal cancer (CRC) have been largely ineffective. We hypothesized that combining an MVA-based vaccine targeting the tumor-associated antigen 5T4 (TroVax) with low-dose cyclophosphamide to deplete Foxp3+regulatory T-cells (Tregs), could improve immunological responses and patient outcomes.
Methods: In this open-label phase I/II clinical trial, TaCTiCC (TroVax and Cyclophosphamide Treatment in Colorectal Cancer) 53 patients with inoperable metastatic CRC were randomized to receive either no treatment (group 1, n=8), metronomic low-dose CPM (50mg B.D. during treatment weeks 1&3; group 2, n=9), TroVax only (6 i.m. injections weeks 4 to 16, group 3, n=18), or low-dose CPM followed by TroVax (group 4, n=18). The primary endpoint was boosted anti-5T4 responses at week 7, as measured by increased T-cell and antibody responses; secondary endpoints included progression-free (PFS)/overall survival (OS), and anti-5T4 responses over the trial period.
Results: CPM depleted Tregs in 21/27 patients during treatment week 3 (p=0.0045), resulting in significantly prolonged PFS amongst groups 2&4 over group 1 (5.0 vs. 2.5 months, HR=0.17 95% CI 0.048-0.62, p=0.0072). TroVax induced a >2-fold increase in anti-5T4 immune responses in 15/36 group 3&4 patients; these patients experienced significantly prolonged median PFS (6.5 vs. 2.4 months, HR 0.31 95% CI 0.14-0.65, p=0.0022) and OS (20 vs. 12 months, HR=0.37 95% CI 0.17-0.82, p=0.014). Combination of CPM & TroVax was not significantly superior. The primary endpoint at a single timepoint was not met since CPM-induced responses declined by week 7, and TroVax-induced responses were greatest at weeks 10-16. No serious adverse events were reported.
Conclusions: Both CPM and TroVax induced highly beneficial anti-tumor immune responses resulting in significantly prolonged survival of end-stage CRC patients without toxicity. This is the first study to show a clear benefit of immunotherapy in advanced CRC, and suggests this approach may be superior (and less toxic) to continuous palliative chemotherapy in these patients. Clinical trial information: 54669986.
darmkanker, chemo, xeloda - capecitabine, overall overleving, ziektevrije tijd, progressie vrije tijd, complementair, immuuntherapie, virus Ankara–5T4, TroVax, cyclophopsphamide
Gerelateerde artikelen
- crispr-cas9-bewerkte T-cellen gericht op CISH maakt patienten met gevorderde darmkanker en GIST alsnog gevoelig voor immuuntherapie met anti-PD medicijnen
- Immuuntherapie met nivolumab plus ipilimumab vooraf aan operatie bij darmkankerpatienten met hoge waarden van microsatellite-instability high/mismatch repair deficient (MSI-H/dMMR) blijkt zeer effectief.
- Immuuntherapie met Pembrolizumab geeft veel betere resultaten op ziektevrije overleving (48 vs 18 procent op 2 jaars meting) dan chemotherapie voor uitgezaaide darmkanker met MSI-H/dMMR - of afwijkende reparatie genen
- KRAS gemuteerde tumoren: Kankervaccin ELI-002 2P stimuleerde hoge T-celreacties bij patiënten met voor immuuntherapie ongevoelige KRAS-gemuteerde tumoren en verbeterde de ziekteprogressieve tijd bij patienten met alvleesklierkanker en darmkanker copy 1 co
- Immuuntherapie vooraf aan operatie en chemotherapie blijkt succesvol bij kankerpatiënten met maagkanker en met tumoren op de overgang van slokdarm naar de maag copy 2
- Dostarlimab, een specifieke vorm van een anti-PD medicijn is 100 procent effectief bij alle 12 patienten met operabele rectumkanker met dMMR = Mismatch-reparatie-deficiëntie en was geen operatie meer nodig.
- Kankerpatienten met solide tumoren met MSI-H = hoge microsatelliet instabiliteit en mismatch reparatie (dMMR) reageren uitstekend op immuuntherapie met pembrolizumab vooraf aan operatie met 65 tot 80 procent complete remissies copy 1
- Immuuntherapie met nivolumab plus ipilimuab voorafgaand aan operatie darmkanker geeft uitstekende resultaten met duurzame klinische complete remissies
- Temozolomide - temodal gevolgd door immunotherapie met combinatie van lage dosis ipilimumab plus nivolumab geeft hoopgevende resultaten bij patiënten met microsatellietstabiel en MGMT-gedempte uitgezaaide darmkanker
- CYAD-101, een vorm van CAR-T cel immuuntherapie geeft hoopvolle resultaten bij uitgezaaide darmkanker zonder dat graft-versus-host-ziekte ontstaat.
- Dendritische cellen en Newcastle Disease Virus bij kankerpatiënten met spijsverteringskanker geeft significant betere resultaten in overlevingstijd aldus gerandomiseerde studie bij 335 patiënten. copy 2
- Autologe genetisch gemodificeerde T-cellen gericht tegen het humaan papillomavirus (HPV) 16 E6 geeft bij patienten met vergevorderde zwaar voorbehandelde uitgezaaide HPV gerelateerde kanker uitstekende resultaten copy 1
- Man met uitgezaaide darmkanker stadium 4 (KRAS pos.) geneest alsnog met combinatie van immuuntherapie met Rigvir virus (dendritische celtherapie) plus FOLFOX en bevacizumab en is nu na 8 jaar kankervrij.
- Nivolumab (Opvido) + ipilimumab (Yervoy) geeft uitstekende resultaten bij nog niet behandelde uitgezaaide darmkanker (met MSI-H of dMMR mutaties) en bereikte 53 procent een gedeeltelijke remissie en 7 procent een complete remissie.
- Immuuntherapie met een gemoduleerd virus plus avelumab, een anti-PD medicijn, wordt onderzocht in een fase I/II studie bij darmkankerpatienten
- Man met uitgezaaide inoperabele darmkanker komt met ATID - Autologous tumor immunizing devascularization, een vorm van immuuntherapie in complete remissie en is al 16 jaar kankervrij, zonder chemo of andere behandelingen
- Immuuntherapie met autovaccinatie van dendritische cellen moet weer eerstelijns behandeling worden voor operabele darmkanker stadium II en III
- Immuuntherapie met dendritische celtherapie geeft uitstekende resultaten op overall overleving en ziektevrije tijd bij darmkanker met weinig of geen zichtbare tumoren.
- Xilonix - MAPp1 zorgt voor stabiele ziekte (bij 53 procent) bij zwaar voorbehandelde darmkankerpatienten stadium 4 met een mediane overall overleving van 4.2 maanden vs 11.5 maanden in vergelijking met placebo copy 1
- Dendritische cellen en Newcastle Disease Virus bij kankerpatiënten met spijsverteringskanker geeft significant betere resultaten in overlevingstijd aldus gerandomiseerde studie bij 335 patiënten. copy 1
- Immuuntherapie: Oncophage(R) (HSPPC-96), gemaakt van eigen tumorcellen zorgt voor opmerkelijke resultaten bij o.a. darmkanker.
- Immuuntherapie met het gemoduleerde virus Ankara–5T4 (TroVax) plus lage dosis cyclophosphamide zorgt voor verdubbeling van mediane overall overleving 11,2 vs 20 maanden bij vergevorderde darmkanker
- Immuuntherapie geeft uitstekende resultaten bij darmkanker met minimale ziekte - weinig tumoren - en zelfs bij darmkanker stadium IV werkt het ook al is het dan minder effectief
- Immuuntherapie met een moleculair middel - codenaam MGN1703 - geeft hoog significant betere ziektevrije tijd bij patiënten met uitgezaaide darmkanker die eerder chemotherapie kregen.
- Autovaccinatie bij darmkanker. Oncovax geeft significant beter resultaat op overleving, overlevingstijd en tijd tot recidief bij darmkankerpatiënten met stadum II. Intracel is failliet verklaard. Oncovax wordt niet meer geleverd
- Archief: immuuntherapie met CEA anti-body plus bestraling geeft hoopvolle resultaten in Phase-II trial met 30 darmkankerpatiënten.
- Immuuntherapie waaronder dendritische celtherapie bij vormen van darmkanker, een overzicht
 Werkingsmechanisme van het Ankara virus (TroVax)
Werkingsmechanisme van het Ankara virus (TroVax)


Plaats een reactie ...
Reageer op "Immuuntherapie met het gemoduleerde virus Ankara–5T4 (TroVax) plus lage dosis cyclophosphamide zorgt voor verdubbeling van mediane overall overleving 11,2 vs 20 maanden bij vergevorderde darmkanker"