Weet dat u als donateur ook korting kunt krijgen op o.a. prostasol bij MEDPRO
18 februari 2026: zie ook dit artikel: https://kanker-actueel.nl/NL/psma-petct-geleide-stereotactische-radiotherapie-bij-prostaatkankerpatienten-geeft-uitstekende-resultaten-op-lange-termijn-waarbij-bijna-driekwart-van-de-patienten-na-5-jaar-vrij-blijft-van-uitzaaiingen-op-afstand.html
13 november 2023: zie ook dit artikel: https://kanker-actueel.nl/theranostiek-succesvol-bij-prostaatkanker-en-schildklierkanker-lijkt-ook-interessante-behandeling-voor-borstkanker-te-kunnen-zijn-aldus-prof-dr-dalm-uit-erasmus-mc.html
1 mei 2020: Aanvullend op onderstaande informatie zie ook dit studierapport: Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study.
PSMA PET / CT werd geassocieerd met een grotere nauwkeurigheid dan conventionele beeldvorming (92% versus 65%). De verbeterde nauwkeurigheid van PSMA PET / CT resulteerde in behandelingswijzigingen en een lagere blootstelling aan straling.
Abstract staat onderaan dit artikel
13 dcecember 2019: Lees ook dit artikel:
15 december 2016: Bron: J Nucl Med. 2016 Nov;57(11):1713-1719. Epub 2016 Jun 3
Veel prostaatkankerpatiënten ervaren progressie van hun ziekte en optredende hormoonresistentie door het oplopen van hun PSA maar vaak zijn in het begin van het oplopen van de PSA nog geen aantoonbare tumoren te vinden. Sinds enkele jaren wordt voor deze groep kankerpatiënten een MP-MRI of PSMA pet-CT scan ingezet.
Een PSMA Pet / CT scan waarbij lutetium 177 wordt gebruikt is tegelijk een behandeling omdat er gericht "bestraald" wordt op een zogeheten PSMA ligand, een eiwit dat gevoelig blijkt voor de radionuclide Gallium 68, een stofje dat gebruikt wordt in de PSMA pet / CT scan en tegelijk met de diagnose (de tumorcellen lichten op) de tumorcellen ook vernietigt.
Zie ook hoe Jacob Zijlstra daarmee zijn prostaatkanker onder controle kreeg.
Tekst gaat onder grafiek verder.
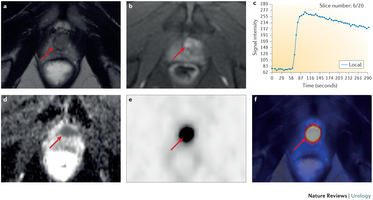
Foto: scanbeelden van 50 jarige prostaatkankerpatient die PSMA scan kreeg.
Mensen met prostaatkanker met een stijgende PSA maar nog geen zichtbare tumoren neem aub contact op met het St. Antonius in Nieuwegein voor een PSMA pet-CT scan.
Citaat van hun website:
Het St. Antonius Ziekenhuis Utrecht/Nieuwegein is het eerste en vooralsnog enige ziekenhuis in Nederland waar het mogelijk is om een PET scan te maken waarmee terugkerende prostaatkanker snel ontdekt kan worden. Het St. Antonius produceert zelf de nieuwe radioligand (radioactieve stof) waarmee al in een zeer vroeg stadium gezien kan worden of er terugkeer is van prostaatkanker (recidief). Hierdoor is snelle behandeling van het recidief mogelijk en dat geeft een betere prognose voor de patiënt.
De nieuwe scan biedt uitkomst voor patiënten die al behandeld zijn voor prostaatkanker (operatie of bestraling) en een verhoogd PSA hebben (PSA is een eiwit in de prostaat; verhoogde PSA-waarden kunnen duiden op prostaatkanker). Bij de eerste verhoging kan de scan, de zogenoemde PSMA PET-scan, al uitgevoerd worden. De scan geeft dan aan welke lymfklieren of andere locaties actief zijn. Voor deze metastasen kan dan naar een gerichte behandeling gezocht worden. Hiermee kan soms verdere uitzaaiing van prostaatkanker voorkomen worden.
Deze vorm van diagnose en tegelijk behandelen zou ook bij andere vormen van kanker kunnen worden ingezet. Zo is er ook een kleine studie gedaan met PSMA aanpak bij uitgezaaide schildkliertumoren en met succes. Zie deze studie: Imaging of Prostate-Specific Membrane Antigen Expression in Metastatic Differentiated Thyroid Cancer Using 68Ga-HBED-CC-PSMA PET/CT.
Hier het persbericht dat ik kreeg toegestuurd:
Identifying and destroying the tumour through nuclear medicine therapy
(Vienna, December 13, 2016) Prostate cancer patients who are resistant to hormone treatment used to have a poor prognosis. Until recently, the diagnostic and therapeutic possibilities had been limited, but now innovative developments in nuclear medicine imaging and therapy open up promising pathways. Novel substances used with PET/CT (positron-emission tomography combined with computed tomography) not only allow for better diagnosis but also offer treatment options where other therapies have failed. "This offers a glimpse of hope to patients who suffer from this particularly severe form of prostate cancer," says EANM expert Prof. Markus Luster.
Prostate cancer is the second most frequently diagnosed cancer in men and causes around 90.000 deaths per year in Europe. Up to every second patient who has his prostate surgically removed or has undergone radiation therapy suffers from relapse. In severe cases the level of testosterone upon which the tumour is dependent to a large extent has to be reduced drastically in order to fight the disease. This is usually done by hormone therapy. However, a considerable number of patients are or will become resistant to this kind of treatment (so called castration-resistant prostate cancer / CRPC). This means that in spite of therapy the tumour has not been destroyed definitively and in many cases is now affecting the lymph nodes or has even extended to the stage of often painful bone metastases. Prognosis of patients who progress to this stage is poor.
Detecting cancer cells by nuclear imaging
A common means to detect prostate cancer and assess the stage of the disease is the measurement of the level of prostate-specific antigen (PSA), which serves as a biomarker for the presence of cancer cells. However, in patients whose testosterone production has been suppressed medically PSA levels are often too low to be measured. This includes CRPC patients in whom this therapy has failed to eradicate or halt the tumour. Moreover, PSA measurement provides no information about the sites and the extent of the recurrent cancer. However, newly developed nuclear medicine methods have opened up promising diagnostic avenues that might more sensitively and accurately enlighten both patient and physician about the location and extent of disease. At the same time, this new approach also provides new therapeutic modalities which can improve the still poor prognosis of CRPC-patients in the future. The leading part is played by a protein called Prostate-Specific Membrane Antigen (PSMA). It is found abundantly on the surface of prostate cancer cells and its number appears to be increasing with the aggressiveness of the disease. This makes PSMA an ideal target for detecting cancer cells by nuclear imaging. The essential means to achieve this is the Ga-68-PSMA-ligand, a substrate that binds to PSMA – comparable to a key that fits into its lock – which is labelled with the radionuclide Gallium 68. This tracer has already been used successfully in a large number of PET/CT examinations: After the patient has been injected with Ga-68-PSMA-ligand the tracer is taken up by the cancer cells which are made visible for the examining physicians by the radiation. "The substance has proven to be highly sensitive and reliable in detecting carcinoma in lymph nodes as well as metastases in other body regions. Over the past decade or so other substances such as choline have been evaluated and applied but in terms of accuracy and diagnostic outcome Ga-68-PSMA is now state of the art," says Prof. Markus Luster.
Tekst gaat verder onder grafiek
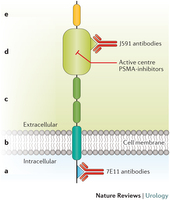
Foto: werkingsmechanisme van PSMA
Combining diagnosis and therapy
As he points out PSMA is not only useful for diagnostic but also for treatment purposes: The PSMA-ligand can be labelled with another radionuclide called Lutetium-177 that is able to destroy the cancer cell from inside through radiation. "Several tests have demonstrated that Lu-177-PSMA-therapy can reduce tumour mass and alleviate pain. Patients who have no other treatment options left and whose cancer cells have been shown to take up PSMA-ligands are very likely to benefit from the diagnostic and therapeutic potential of PSMA imaging and therapy," says Prof. Markus Luster.
Zoals eerder gezegd, in Nederland is een PSMA behandeling aan te vragen en te verkrijgen in het St. Antonius in Nieuwegein voor een PSMA pet-CT scan.
Bronnen voor bovenstaand artikel: www.eanm.org/content-eanm/uploads/2016/11/201612_EANM_Prostate-cancer_en.pdf
En www.facebook.com/officialEANM.
En www.whatisnuclearmedicine.com
Press contact
impressum health & science communication
Frank von Spee
E-Mail: vonspee@impressum.de
Tel: +49 (0)40 - 31 78 64 10
impressum health & science communication
Registered Office: Hamburg
Register Court: Local Court (Amtsgericht) Hamburg
Register Number: HRA 92757
Tax Number: 22/230/20405
Managing Partners:
Henry Friedrich Meyer
Frank von Spee
Een reviewstudie die bovenstaand persbericht bevestigt is dit studierapport met lange referentielijst dat tegen betaling is in te zien:
Current use of PSMA–PET in prostate cancer management
Hier het abstract van deze review studie:
PSMA-based imaging holds great promise to improve prostate cancer management.
Current use of PSMA–PET in prostate cancer management
- Nature Reviews Urology
- 13,
- 226–235
- doi:10.1038/nrurol.2016.26
- Published online
Abstract
Currently, the findings of imaging procedures used for detection or staging of prostate cancer depend on morphology of lymph nodes or bone metabolism and do not always meet diagnostic needs. Prostate-specific membrane antigen (PSMA), a transmembrane protein that has considerable overexpression on most prostate cancer cells, has gained increasing interest as a target molecule for imaging. To date, several small compounds for labelling PSMA have been developed and are currently being investigated as imaging probes for PET with the 68Ga-labelled PSMA inhibitor Glu-NH-CO-NH-Lys(Ahx)-HBED-CC being the most widely studied agent. 68Ga-PSMA–PET imaging in combination with multiparametric MRI (mpMRI) might provide additional molecular information on cancer localization within the prostate. In patients with primary prostate cancer of intermediate-risk to high-risk, PSMA-based imaging has been reported to improve detection of metastatic disease compared with CT or mpMRI, rendering additional cross-sectional imaging or bone scintigraphy unnecessary. Furthermore, in patients with biochemically recurrent prostate cancer, use of 68Ga-PSMA–PET imaging has been shown to increase detection of metastatic sites, even at low serum PSA values, compared with conventional imaging or PET examination with different tracers. Thus, although current knowledge is still limited and derived mostly from retrospective series, PSMA-based imaging holds great promise to improve prostate cancer management.
REferences:
References
- et al. Global cancer statistics, 2012. CA Cancer J. Clin. 65, 87–108 (2015).
- American Urological Association. Guideline for the management of clinically localized prostate cancer (2007). , (2007).
- European Association of Urology. Guidelines on Prostate Cancer. http://uroweb.org/guideline/prostate-cancer/ (2015).
- et al. ESUR prostate MR guidelines 2012. Eur. Radiol. 22, 746–757 (2012).
- et al. Magnetic resonance imaging-transectal ultrasound image-fusion biopsies accurately characterize the index tumor: correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur. Urol. 67, 787–794 (2015).
- et al. Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. Eur. Urol. 68, 8–19 (2015).
- , & Performance of multiparametric magnetic resonance imaging in the evaluation and management of clinically low-risk prostate cancer. Urol. Oncol. 32, 39.e1–39.e10 (2014).
- et al. 1.5-T multiparametric MRI using PI-RADS: a region by region analysis to localize the index-tumor of prostate cancer in patients undergoing prostatectomy. Acta Radiol. 56, 500–511 (2015).
- et al. Transition zone prostate cancer: detection and localization with 3-T multiparametric MR imaging. Radiology 266, 207–217 (2013).
- et al. MR-sequences for prostate cancer diagnostics: validation based on the PI-RADS scoring system and targeted MR-guided in-bore biopsy. Eur. Radiol. 24, 2582–2589 (2014).
- National Comprehensive Cancer Network. Prostate cancer. ">, (2015).
- , , , & The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur. Urol. 64, 106–117 (2013).
- , , , & Comparative performance of PET tracers in biochemical recurrence of prostate cancer: a critical analysis of literature. Am. J. Nucl. Med. Mol. Imaging 4, 580–601 (2014).
- et al. The sensitivity of [11C]choline PET/CT to localize prostate cancer depends on the tumor configuration. Clin. Cancer Res. 17, 3751–3759 (2011).
- , & 18F-choline, 11C-choline and 11C-acetate PET/CT: comparative analysis for imaging prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 40, S18–S27 (2013).
- , , , & Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur. Urol. 63, 1040–1048 (2013).
- , , & A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin. Oncol. (R. Coll. Radiol.) 22, 46–55 (2010).
- et al. The detection rate of [11C]choline-PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 35, 18–23 (2008).
- et al. 2-[18F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin. Cancer Res. 11, 4761–4769 (2005).
- , , , & [68Ga]gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur. J. Nuclear Med. Mol. Imaging 39, 1085–1086 (2012).
- et al. Prostate-specific membrane antigen: evidence for the existence of a second related human gene. Br. J. Cancer 72, 583–588 (1995).
- et al. Mapping, genomic organization and promoter analysis of the human prostate-specific membrane antigen gene. Biochim. Biophys. Acta 1443, 113–127 (1998).
- , , & Pathological and molecular aspects of prostate cancer. Lancet 361, 955–964 (2003).
- et al. Identification, purification, and subcellular localization of prostate-specific membrane antigen PSM′ protein in the LNCaP prostatic carcinoma cell line. Cancer Res. 58, 4787–4789 (1998).
- Characterization and glutamyl preferring carboxypeptidase function of prostate specific membrane antigen: a novel folate hydrolase. Urology 49, 104–112 (1997).
- , , , & Prostate adenocarcinoma presenting with inguinal lymphadenopathy. Urology 61, 463 (2003).
- , , , & The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. AJR Am. J. Roentgenol. 199, 103–110 (2012).
- et al. Tumour markers for managing men who present with metastatic prostate cancer and serum prostate-specific antigen levels of <10 ng/mL. BJU Int. 96, 303–307 (2005).
- et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc. Natl Acad. Sci. USA 108, 9578–9582 (2011).
- et al. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin. Cancer Res. 5, 2674–2681 (1999).
- et al. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 59, 3192–3198 (1999).
- , , & Metastatic renal cell carcinoma neovasculature expresses prostate-specific membrane antigen. Urology 57, 801–805 (2001).
- et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum. Pathol. 40, 1754–1761 (2009).
- , , , & Folate hydrolase (prostate-specific membrane antigen) 1 expression in bladder cancer subtypes and associated tumor neovasculature. Mod. Pathol. 24, 1521–1529 (2011).
- , , , & Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 3, 81–85 (1997).
- , & Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc. Natl Acad. Sci. USA 93, 749–753 (1996).
- , , & Hydrolysis of the brain dipeptide N-acetyl-l-aspartyl-l-glutamate. J. Biol. Chem. 262, 14498–14506 (1987).
- et al. Detection of 18F-FDG PET/CT occult lesions with 18F-DCFPyL PET/CT in a patient with metastatic renal cell carcinoma. Clin. Nucl. Med. 41, 83–85 (2015).
- et al. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted F-DCFPyL PET/CT. Ann. Nucl. Med. 29, 877–882 (2015).
- , , , & First evidence of PSMA expression in differentiated thyroid cancer using [68Ga]PSMA-HBED-CC PET/CT. Eur. J. Nucl. Med. Mol. Imaging 42, 1622–1623 (2015).
- et al. [68Ga]PSMA-HBED uptake mimicking lymph node metastasis in coeliac ganglia: an important pitfall in clinical practice. Eur. J. Nucl. Med. Mol. Imaging 42, 210–214 (2015).
- et al. In vivo visualization of prostate-specific membrane antigen in glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 42, 170–171 (2015).
- , , & Intense PSMA-expression using 68Ga-PSMA PET/CT in a paravertebral schwannoma mimicking prostate cancer metastasis. Eur. J. Nucl. Med. Mol. Imaging 43, 193–194 (2016).
- & Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell. Biochem. 91, 528–539 (2004).
- et al. The homodimer of prostate-specific membrane antigen is a functional target for cancer therapy. Proc. Natl Acad. Sci. USA 100, 12590–12595 (2003).
- & Degradation of halogenated aromatic compounds. Biodegradation 1, 207–220 (1990).
- , , , & Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer 82, 2256–2261 (1998).
- et al. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol. Oncol. Res. 15, 167–172 (2009).
- , & Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int. J. Cancer. 62, 552–558 (1995).
- et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur. Urol. 68, 530–534 (2015).
- Overview of prostate-specific membrane antigen. Rev. Urol. 6, S13–S18 (2004).
- et al. A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen. Mol. Biol. Cell 14, 4835–4845 (2003).
- et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 58, 4055–4060 (1998).
- et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug. Chem. 23, 688–697 (2012).
- & Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell. Biochem. 91, 528–539 (2004).
- , & Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate 30, 232–242 (1997).
- et al. Anti-prostate-specific membrane antigen-based radioimmunotherapy for prostate cancer. Cancer 116, 1075–1083 (2010).
- et al. PET imaging of prostate cancer xenografts with a highly specific antibody against the prostate-specific membrane antigen. J. Nuclear Med. 50, 606–611 (2009).
- et al. 89Zr-DFO-J591 for immunoPET of prostate-specific membrane antigen expression in vivo. J. Nucl. Med. 51, 1293–1300 (2010).
- et al. Pharmacokinetics and PET imaging properties of two recombinant anti-PSMA antibody fragments in comparison to their parental antibody. Prostate 74, 743–755 (2014).
- , , & Molecular characterization of human brain N-acetylated α-linked acidic dipeptidase (NAALADase). J. Pharmacol. Exp. Ther. 286, 1020–1025 (1998).
- , , , & Characterization of the enzymatic activity of PSM: comparison with brain NAALADase. Prostate 39, 28–35 (1999).
- et al. Bioisosterism of urea-based GCPII inhibitors: synthesis and structure–activity relationship studies. Bioorg. Med. Chem. Lett. 20, 392–397 (2010).
- , & PET imaging in prostate cancer: focus on prostate-specific membrane antigen. Curr. Top. Med. Chem. 13, 951–962 (2013).
- et al. Radiolabeled small-molecule ligands for prostate-specific membrane antigen: in vivo imaging in experimental models of prostate cancer. Clin. Cancer Res. 11, 4022–4028 (2005).
- et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 69, 6932–6940 (2009).
- et al. A series of halogenated heterodimeric inhibitors of prostate specific membrane antigen (PSMA) as radiolabeled probes for targeting prostate cancer. J. Med. Chem. 52, 347–357 (2009).
- , , , & Design, synthesis, and preclinical evaluation of prostate-specific membrane antigen targeted 99mTc-radioimaging agents. Mol. Pharm. 6, 790–800 (2009).
- et al. Synthesis and SAR of 99mTc/Re-labeled small molecule prostate specific membrane antigen inhibitors with novel polar chelates. Bioorg. Med. Chem. Lett. 23, 1557–1563 (2013).
- et al. 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin. Cancer Res. 17, 7645–7653 (2011).
- et al. 18F-DCFBC PET/CT for PSMA-based detection and characterization of primary prostate cancer. J. Nucl. Med. 56, 1003–1010 (2015).
- et al. A modular strategy to prepare multivalent inhibitors of prostate-specific membrane antigen (PSMA). Oncotarget 2, 1244–1253 (2011).
- et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 42, 197–209 (2015).
- et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 41, 11–20 (2014).
- et al. PET/MRI with a 68Ga-PSMA ligand for the detection of prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 40, 1629–1630 (2013).
- , , , & Synthesis and preclinical evaluation of DOTAGA-conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer. EJNMMI Res. 4, 63 (2014).
- et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 40, 486–495 (2013).
- & The renaissance of the 68Ge/68Ga radionuclide generator initiates new developments in 68Ga radiopharmaceutical chemistry. Curr. Top. Med. Chem. 10, 1633–1668 (2010).
- , , , & [111In]PSMA-I&T: expanding the spectrum of PSMA-I&T applications towards SPECT and radioguided surgery. EJNMMI Res. 5, 68 (2015).
- et al. 68Ga- and 177Lu-Labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J. Nucl. Med. 56, 1169–1176 (2015).
- , , , & Synthesis and preclinical evaluation of DOTAGA-conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer. EJNMMI Res. 4, 63 (2014).
- et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J. Nucl. Med. 56, 914–920 (2015).
- et al. The novel theranostic PSMA-ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry and first evaluation of tumor lesions. J. Nucl. Med. 56, 1697–1705 (2015).
- et al. Dosimetry for Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 43, 42–51 (2015).
- et al. Early side effects and first results of radioligand therapy with 177Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 5, 114 (2015).
- et al. Comparison of [18F]DCFPyL and [68Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol. Imaging Biol. 17, 575–584 (2015).
- et al. Radiofluorination of PSMA-HBED via Al18F2+ chelation and biological evaluations in vitro. Mol. Imaging Biol. 17, 777–785 (2015).
- et al. N-[N-[(S)-1,3-Dicarboxypropyl]carbamoyl]-4-[18F]fluorobenzyl-l-cysteine, [18F]DCFBC: a new imaging probe for prostate cancer. Clin. Cancer Res. 14, 3036–3043 (2008).
- et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J. Nucl. Med. 53, 1883–1891 (2012).
- et al. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol. Imaging Biol. 17, 565–574 (2015).
- et al. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology 241, 449–458 (2006).
- , , & Prostate cancer: apparent diffusion coefficient map with T2-weighted images for detection — a multireader study. Radiology 250, 145–151 (2009).
- et al. Value of magnetic resonance spectroscopy imaging and dynamic contrast-enhanced imaging for detecting prostate cancer foci in men with prior negative biopsy. Clin. Cancer Res. 16, 1875–1883 (2010).
- , , & Diffusion-weighted MRI in the detection of prostate cancer: meta-analysis. AJR Am. J. Roentgenol. 199, 822–829 (2012).
- In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissues using echo-planar imaging. J. Magn. Reson. Imaging 16, 196–200 (2002).
- , , & Diffusion-weighted imaging with apparent diffusion coefficient mapping and spectroscopy in prostate cancer. Top. Magn. Reson. Imaging 19, 261–272 (2008).
- et al. In vivo measurement of the apparent diffusion coefficient in normal and malignant prostatic tissue using thin-slice echo-planar imaging. Radiol. Med. 111, 1124–1133 (2006).
- et al. Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology 74, 1094–1099 (2009).
- et al. Washout gradient in dynamic contrast-enhanced MRI is associated with tumor aggressiveness of prostate cancer. J. Magn. Reson. Imaging 36, 912–919 (2012).
- et al. Diffusion-weighted and dynamic contrast-enhanced MRI of prostate cancer: correlation of quantitative MR parameters with Gleason score and tumor angiogenesis. AJR Am. J. Roentgenol. 197, 1382–1390 (2011).
- et al. Low suspicion lesions on multiparametric magnetic resonance imaging predict for the absence of high-risk prostate cancer. BJU Int. 110, E783–E788 (2012).
- et al. PET/MR in prostate cancer: technical aspects and potential diagnostic value. Eur. J. Nucl. Med. Mol. Imaging 40, S79–S88 (2013).
- et al. Comparison of integrated whole-body [11C]choline PET/MR with PET/CT in patients with prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 40, 1486–1499 (2013).
- et al. 68Ga-PSMA PET/MR with multimodality image analysis for primary prostate cancer. Abdom. Imaging 40, 1769–1771 (2014).
- et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur. Urol.(in the press).
- et al. PSMA-PET/MRI-guided transrectal fusion biopsy for the detection of prostate cancer. Eur. Urol. Suppl. 14, E217 (2015).
- et al. Multimodal image-guided prostate fusion biopsy based on automatic deformable registration. Int. J. Comput. Assist. Radiol. Surg. 10, 1997–2007 (2015).
- et al. Diagnostic efficacy of 68Gallium-PSMA-PET compared to conventional imaging in lymph node staging of of 130 consecutive patients with intermediate to high-risk prostate cancer. J. Urol. http://dx.doi.org/10.1016/j.juro.2015.12.025 (2015).
- et al. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur. Urol. 62, 68–75 (2012).
- et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 9, 850–856 (2008).
- et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin. Radiol. 63, 387–395 (2008).
- et al. Comparison between malignant and benign abdominal lymph nodes on diffusion-weighted imaging. Acad. Radiol. 15, 641–646 (2008).
- et al. Preliminary results for characterization of pelvic lymph nodes in patients with prostate cancer by diffusion-weighted MR-imaging. Invest. Radiol. 45, 15–23 (2010).
- , , , & Feasibility of diffusion-weighted imaging in the differentiation of metastatic from nonmetastatic lymph nodes: early experience. J. Magn. Reson. Imaging 28, 714–719 (2008).
- Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: utility and limitations. Eur. J. Nucl. Med. Mol. Imaging 40, S5–S10 (2013).
- et al. Application of C-11-acetate positron-emission tomography (PET) imaging in prostate cancer: systematic review and meta-analysis of the literature. BJU Int. 112, 1062–1072 (2013).
- et al. Comparative effectiveness of [18F]-fluorocholine PET-CT and pelvic MRI with diffusion-weighted imaging for staging in patients with high-risk prostate cancer. Prostate 75, 323–331 (2015).
- , , , & [11C]acetate positron emission tomography-computed tomography imaging of prostate cancer lymph-node metastases correlated with histopathological findings after extended lymphadenectomy. Scand. J. Urol. 49, 35–42 (2015).
- , , , & Preoperative staging of pelvic lymph nodes in prostate cancer by 11C-choline PET. J. Nucl. Med. 44, 331–335 (2003).
- et al. Experience with carbon-11 choline positron emission tomography in prostate carcinoma. Eur. J. Nucl. Med. 27, 1415–1419 (2000).
- et al. 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology 254, 925–933 (2010).
- et al. 18F-fluorocholine PET/CT compared with extended pelvic lymph node dissection in high-risk prostate cancer. World J. Urol. 32, 965–970 (2014).
- , , , & Detection of brain metastasis with 68Ga-labeled PSMA ligand PET/CT: a novel radiotracer for imaging of prostate carcinoma. Clin. Nucl. Med. 40, 328–329 (2015).
- et al. Evaluation of PSMA PET/CT imaging using a 68Ga-HBED-CC ligand in patients with prostate cancer and the value of early pelvic imaging. Nucl. Med. Commun. 36, 582–587 (2015).
- et al. Positron emission tomography/magnetic resonance imaging with 68Gallium-labeled ligand of prostate-specific membrane antigen: promising novel option in prostate cancer imaging? Int. J. Urol. 21, 1286–1288 (2014).
- et al. Early salvage radiotherapy following radical prostatectomy. Eur. Urol. 65, 1034–1043 (2014).
- The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int. J. Radiat. Oncol. Biol. Phys. 84, 104–111 (2012).
- , & Imaging of prostate cancer local recurrences: why and how? Eur. Radiol 20, 1254–1266 (2010).
- et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J. Nucl. Med. 56, 668–674 (2015).
- et al. Is there a role for 11C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase <1.5 ng/ml? Eur. J. Nucl. Med. Mol. Imaging 38, 55–63 (2011).
- & 11C-choline PET/CT and PSA kinetics. Eur. J. Nucl. Med. Mol. Imaging 40, S36–S40 (2013).
- et al. Relationship between PSA kinetics and [18F]fluorocholine PET/CT detection rates of recurrence in patients with prostate cancer after total prostatectomy. Eur. J. Nucl. Med. Mol. Imaging 39, 271–282 (2012).
- et al. PET imaging with 68Gallium-labelled ligand of prostate-specific membrane antigen (68Ga-HBED-PSMA) for staging of biochemical recurrent prostate cancer after radical prostatectomy. J. Clin. Oncol. 33 (Suppl.),5023 (2015).
- US National Library of Medicine. ClinicalTrials.gov , (2015).
- US National Library of Medicine. ClinicalTrials.gov , (2015).
- US National Library of Medicine. ClinicalTrials.gov , (2015).
- EU Clinical Trials Register. clinicaltrialsregister.eu , (2015).
PSMA PET-CT is a suitable replacement for conventional imaging, providing superior accuracy, to the combined findings of CT and bone scanning.
Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study.
Abstract
BACKGROUND:
Conventional imaging using CT and bone scan has insufficient sensitivity when staging men with high-risk localised prostate cancer. We aimed to investigate whether novel imaging using prostate-specific membrane antigen (PSMA) PET-CT might improve accuracy and affect management.
METHODS:
In this multicentre, two-arm, randomised study, we recruited men with biopsy-proven prostate cancer and high-risk features at ten hospitals in Australia. Patients were randomly assigned to conventional imaging with CT and bone scanning or gallium-68 PSMA-11 PET-CT. First-line imaging was done within 21 days following randomisation. Patients crossed over unless three or more distant metastases were identified. The primary outcome was accuracy of first-line imaging for identifying either pelvic nodal or distant-metastatic disease defined by the receiver-operating curve using a predefined reference-standard including histopathology, imaging, and biochemistry at 6-month follow-up. This trial is registered with the Australian New Zealand Clinical Trials Registry, ANZCTR12617000005358.
FINDINGS:
From March 22, 2017 to Nov 02, 2018, 339 men were assessed for eligibility and 302 men were randomly assigned. 152 (50%) men were randomly assigned to conventional imaging and 150 (50%) to PSMA PET-CT. Of 295 (98%) men with follow-up, 87 (30%) had pelvic nodal or distant metastatic disease. PSMA PET-CT had a 27% (95% CI 23-31) greater accuracy than that of conventional imaging (92% [88-95] vs 65% [60-69]; p<0·0001). We found a lower sensitivity (38% [24-52] vs 85% [74-96]) and specificity (91% [85-97] vs 98% [95-100]) for conventional imaging compared with PSMA PET-CT. Subgroup analyses also showed the superiority of PSMA PET-CT (area under the curve of the receiver operating characteristic curve 91% vs 59% [32% absolute difference; 28-35] for patients with pelvic nodal metastases, and 95% vs 74% [22% absolute difference; 18-26] for patients with distant metastases). First-line conventional imaging conferred management change less frequently (23 [15%] men [10-22] vs 41 [28%] men [21-36]; p=0·008) and had more equivocal findings (23% [17-31] vs 7% [4-13]) than PSMA PET-CT did. Radiation exposure was 10·9 mSv (95% CI 9·8-12·0) higher for conventional imaging than for PSMA PET-CT (19·2 mSv vs 8·4 mSv; p<0·001). We found high reporter agreement for PSMA PET-CT (κ=0·87 for nodal and κ=0·88 for distant metastases). In patients who underwent second-line image, management change occurred in seven (5%) of 136 patients following conventional imaging, and in 39 (27%) of 146 following PSMA PET-CT.
INTERPRETATION:
PSMA PET-CT is a suitable replacement for conventional imaging, providing superior accuracy, to the combined findings of CT and bone scanning.
FUNDING:
Movember and Prostate Cancer Foundation of Australia. VIDEO ABSTRACT.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Comment in
- PMID:
- 32209449
- DOI:
- 10.1016/S0140-6736(20)30314-7
- [Indexed for MEDLINE]
Gerelateerde artikelen
- PSMA PET/CT-geleide stereotactische radiotherapie bij prostaatkankerpatienten geeft uitstekende resultaten op lange termijn, waarbij bijna driekwart van de patiënten na 5 jaar vrij blijft van uitzaaiingen op afstand
- 177Lu-PSMA-617 naast hormoontherapie plus een hormoonreceptorremmer (ARPI) verlengt aantoonbaar de ziekteprogressievrije overleving bij mannen met uitgezaaide hormoongevoelige prostaatkanker
- 177Lu-PSMA-617 geeft betere progressievrije overleving en algehele overleving naast standaardzorg bij patiënten met gevorderde PSMA-positieve gemetastaseerde castratieresistente prostaatkanker
- lutetium-177 PSMA-617 gerichte bestralingsbehandeling geeft verdubbeling van ziekteprogressievrije tijd in vergelijking met abiraterone of enzalutamide bij patienten met uitgezaaide hormoonresistente prostaatkanker die nog geen chemo hadden gehad
- lutetium-177 PSMA-617 gerichte bestralingsbehandeling geeft alsnog uitstekende resultaten op overall overleving (plus 33 maanden) bij patienten met uitgezaaide hormoonresistente prostaatkanker.
- PSMA - Prostate Specific Membrane Antigen samen met pet / CT scan blijkt succesvolle behandeling voor hormoonresistente prostaatkankerpatienten met oplopende PSA
- PSMA gerichte PET scan bij mannen met verhoogde PSA is effectieve manier in ontdekken van plaats van tumoren. Bij uitzaaiingen op afstand heeft bestralen van bekkengebied geen zin.



Plaats een reactie ...
1 Reactie op "PSMA - Prostate Specific Membrane Antigen samen met pet / CT scan blijkt succesvolle behandeling voor hormoonresistente prostaatkankerpatienten met oplopende PSA"