Zie ook in gerelateerde artikelen
28 januari 2026: Bron: FDA o.a.
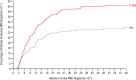
Wanneer daramutumab (Darazalex) en hyaluronidase-fihj (Darzalex Faspro) in combinatie met bortezomib (Velcade), lenalidomide (Revlimid) en dexamethason als behandeling wordt gegeven bij patiënten met een eerste diagnose van Multiple Myeloma (botkanker, ziekte van Kahler) die niet in aanmerking komen voor een autologe stamceltransplantatie dan blijkt de ziekteprogressievrije tijd en overall overleving aanzienlijk beter dan met alleen daramutumab (Darazalex) aanvullend op bortezomib (Velcade), lenalidomide (Revlimid) en alleen dexamethason in de combinatiebehandeling.
De resultaten uit de fase 3 studie CEPHEUS zijn, vertaalt in het Nederlands:
In de daramutumab (Darazalex) en hyaluronidase-fihj (Darzalex Faspro) groep bedroeg de mediane behandelingsduur 56,3 maanden (bereik: 0,1-64,6) versus 34,3 maanden (bereik: 0,5-63,8) met alleen dexamethason in de combinatiebehandeling.²
Bij patiënten in de daramutumab (Darazalex) en hyaluronidase-fihj (Darzalex Faspro) groep met een complete respons (CR) of beter (n = 150) was het percentage MRD-negativiteit 68,7% (95% betrouwbaarheidsinterval: 60,6%-76,0%).
Bij patiënten in de controlegroep die een complete respons (CR) of beter bereikten (n = 116) was dit percentage 59,5% (95% betrouwbaarheidsinterval: 50,0%-68,5%).
De percentages aanhoudende MRD-negativiteit in deze respectievelijke groepen waren 42,6% versus 25,3% (p = 0,0003).
In de daramutumab (Darazalex) en hyaluronidase-fihj (Darzalex Faspro) groep was de objectieve responsratio (ORR) 97,0%, inclusief strikte complete respons (sCR's; 65,0%), complete respons (CR's; 16,2%), zeer goede partiële responsen (VGPR's; 11,7%) en partiële responsen (PR's; 4,1%).
In de groep met alleen daramutumab (Darazalex) was de ORR 93,4%, inclusief sCR's (44,9%), CR's (16,7%), VGPR's (24,7%) en PR's (7,1%).
Op basis van deze resultaten heeft de FDA - Food and Drug Administration deze behandeling goedgekeurd om als eerstelijns behandeling te gebruiken bij deze groep van patiënten. Zie dit artikel op de website van de FDA.
Eerder werden de resultaten van de fase III studie CEPHEUS gepubliceerd:
Daratumumab plus bortezomib, lenalidomide and dexamethasone for transplant-ineligible or transplant-deferred newly diagnosed multiple myeloma: the randomized phase 3 CEPHEUS trial
- PMID: 39910273
- PMCID: PMC12003169
- DOI: 10.1038/s41591-024-03485-7
Erratum in
-
Author Correction: Daratumumab plus bortezomib, lenalidomide and dexamethasone for transplant-ineligible or transplant-deferred newly diagnosed multiple myeloma: the randomized phase 3 CEPHEUS trial.Nat Med. 2025 Apr;31(4):1366. doi: 10.1038/s41591-025-03581-2.PMID: 39948407 Free PMC article. No abstract available.
Abstract
Frontline daratumumab-based triplet and quadruplet standard-of-care regimens have demonstrated improved survival outcomes in newly diagnosed multiple myeloma (NDMM). For patients with transplant-ineligible NDMM, triplet therapy with either daratumumab plus lenalidomide and dexamethasone (D-Rd) or bortezomib, lenalidomide and dexamethasone (VRd) is the current standard of care. This phase 3 trial evaluated subcutaneous daratumumab plus VRd (D-VRd) in patients with transplant-ineligible NDMM or for whom transplant was not planned as the initial therapy (transplant deferred). Some 395 patients with transplant-ineligible or transplant-deferred NDMM were randomly assigned to eight cycles of D-VRd or VRd followed by D-Rd or Rd until progression. The primary endpoint was overall minimal residual disease (MRD)-negativity rate at 10-5 by next-generation sequencing. Major secondary endpoints included complete response (CR) or better (≥CR) rate, progression-free survival and sustained MRD-negativity rate at 10-5. At a median follow-up of 58.7 months, the MRD-negativity rate was 60.9% with D-VRd versus 39.4% with VRd (odds ratio, 2.37; 95% confidence interval (CI), 1.58-3.55; P < 0.0001). Rates of ≥CR (81.2% versus 61.6%; P < 0.0001) and sustained MRD negativity (≥12 months; 48.7% versus 26.3%; P < 0.0001) were significantly higher with D-VRd versus VRd. Risk of progression or death was 43% lower for D-VRd versus VRd (hazard ratio, 0.57; 95% CI, 0.41-0.79; P = 0.0005). Adverse events were consistent with the known safety profiles for daratumumab and VRd. Combining daratumumab with VRd produced deeper and more durable MRD responses versus VRd alone. The present study supports D-VRd quadruplet therapy as a new standard of care for transplant-ineligible or transplant-deferred NDMM. ClinicalTrials.gov registration: NCT03652064 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.Z.U. received research funding from Amgen, Array BioPharma, Bristol Myers Squibb, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDx and Takeda and consulted for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Edo Pharma, Genentech, Gilead, GSK, Janssen, Oncopeptides, Sanofi, Seattle Genetics, Secura Bio, SkylineDx, Takeda and TeneoBio. V.H. received honoraria for lectures/advisory boards from AbbVie, Amgen, Bristol Myers Squibb, GSK, Johnson & Johnson, Pfizer, Regeneron, Sanofi and Takeda. N.J.B. consulted for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Janssen, GSK, Genentech, Karyopharm Therapeutics, Kite, Novartis, Pfizer, Roche, Sanofi and Takeda, received research funding from Janssen and Pfizer and honoraria from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Janssen, GSK, Genentech, Karyopharm Therapeutics, Kite, Novartis, Pfizer, Roche, Sanofi and Takeda and served on the Board of Directors or advisory committees for AbbVie, Amgen, Bristol Myers Squibb, Celgene, Janssen, GSK, Genentech, Karyopharm Therapeutics, Kite, Novartis, Pfizer, Roche, Sanofi and Takeda. C.P.V. received honoraria from Janssen, Bristol Myers Squibb, GSK, Sanofi, Pfizer, AbbVie and Forus. M. Braunstein participated in speakers bureaus for Bristol Myers Squibb, Janssen, Takeda and Sanofi. J.M.M. served as a consultant for Bristol Myers Squibb, Medison Pharma, Pfizer and Roche. Y.C.C. served as a consultant for, received research funding and honoraria from and served on the Board of Directors or advisory committees for Bristol Myers Squibb, Janssen, Takeda, Sanofi and GSK. M.M. received honoraria from Janssen Pharmaceuticals, Ono Pharmaceutical, Takeda Pharmaceuticals, Sanofi K.K. Nippon Kayaku and SymBio Pharmaceuticals and received research funding from Janssen Pharmaceuticals, Bristol Myers Squibb K.K., GSK and Pfizer. K.S. received lecture fees from Takeda, Ono Pharmaceutical, Novartis, Sanofi, Bristol Myers Squibb and Janssen and received advisory fees from SRL. M. Beksac served as a consultant for Bristol Myers Squibb, Takeda, Janssen, Menarini, Amgen and GSK and participated in speakers bureaus for Bristol Myers Squibb, Janssen, Takeda and Sanofi. A.M. served as a consultant for Janssen, Takeda, Amgen, Bristol Myers Squibb, Sanofi, Novartis, AstraZeneca, Pfizer and AbbVie and received honoraria from Janssen, Takeda, Amgen, Bristol Myers Squibb, Sanofi, Novartis, AstraZeneca, Pfizer and AbbVie. H.T. served as a consultant for SRL, received honoraria from Janssen, Ono Pharmaceutical, Sanofi and Bristol Myers Squibb and received research funding from Bristol Myers Squibb. A.P. received research funding from Bristol Myers Squibb, Sanofi and Takeda. T.A. is an employee of Genmab and owns stock. W. Liu, J.W., K.C., J.V., M.K., L.L.-M., J.C., M.R. and R.C. are employees of Janssen. S.Z. received research funding from Janssen and Takeda and participated in advisory boards (fees to institute) for Janssen, Bristol Myers Squibb, Sanofi, Oncopeptides, Amgen and Takeda. The other authors declare no competing interests.
Similar articles
-
Induction therapy with bortezomib, melphalan, and prednisone followed by lenalidomide and dexamethasone versus carfilzomib, lenalidomide, and dexamethasone with or without daratumumab in older, fit patients with newly diagnosed multiple myeloma (GEM-2017FIT): a phase 3, open-label, multicentre, randomised clinical trial.Lancet Haematol. 2025 Aug;12(8):e588-e598. doi: 10.1016/S2352-3026(25)00143-7.PMID: 40769684Clinical Trial.
-
Isatuximab, lenalidomide, dexamethasone and bortezomib in transplant-ineligible multiple myeloma: the randomized phase 3 BENEFIT trial.Nat Med. 2024 Aug;30(8):2235-2241. doi: 10.1038/s41591-024-03050-2. Epub 2024 Jun 3.PMID: 38830994Free PMC article.Clinical Trial.
-
Survival impact of anti-CD38-based quadruplet regimens in transplant-ineligible newly diagnosed multiple myeloma: a network meta-analysis and reconstructed individual patient data meta-analysis.Blood Cancer J. 2025 Dec 6;15(1):212. doi: 10.1038/s41408-025-01413-7.PMID: 41353340Free PMC article.
-
Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma.N Engl J Med. 2024 Jan 25;390(4):301-313. doi: 10.1056/NEJMoa2312054. Epub 2023 Dec 12.PMID: 38084760Clinical Trial.
-
Daratumumab, Lenalidomide, and Dexamethasone Versus Bortezomib, Lenalidomide, and Dexamethasone in Transplant-Ineligible Newly Diagnosed Multiple Myeloma: A Systematic Literature Review and Meta-Analysis.Hematol Oncol. 2025 May;43(3):e70061. doi: 10.1002/hon.70061.PMID: 40207704Free PMC article.
Cited by
-
A multicenter observational retrospective study of second-line treatment with daratumumab-bortezomib-dexamethasone (DaraVd) in multiple myeloma patients refractory to lenalidomide.Clin Exp Med. 2025 Nov 25;26(1):43. doi: 10.1007/s10238-025-01956-w.PMID: 41286382Free PMC article.
-
A network meta-analysis of randomized clinical trials in lenalidomide-exposed or -refractory multiple myeloma patients.ESMO Open. 2025 Aug;10(8):105514. doi: 10.1016/j.esmoop.2025.105514. Epub 2025 Jul 15.PMID: 40669094Free PMC article.
-
Correction: Cost of Anti-CD38 Monoclonal Antibodies in Combination With Bortezomib, Lenalidomide and Dexamethasone for the Frontline Treatment of Transplant-Ineligible Patients With Newly Diagnosed Multiple Myeloma in the US.J Health Econ Outcomes Res. 2025 Aug 12;12(2):62-66. doi: 10.36469/001c.143106. eCollection 2025.PMID: 40822986Free PMC article.
-
High Implementation Adherence to Lenalidomide in Multiple Myeloma.Cancers (Basel). 2025 Nov 6;17(21):3587. doi: 10.3390/cancers17213587.PMID: 41228378Free PMC article.
-
Bortezomib-melphalan-prednisone <I>versus</i> lenalidomide-dexamethasone in elderly patients with multiple myeloma: the Real MM phase IV trial.Haematologica. 2026 Jan 1;111(1):361-367. doi: 10.3324/haematol.2025.287510. Epub 2025 Jul 31.PMID: 40820797Free PMC article.No abstract available.
References
- Lammerts van Bueren, J. et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood124, 3474 (2014). - DOI
- Moreau, P. et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab and followed by daratumumab maintenance or observation in transplant-eligible newly diagnosed multiple myeloma: long-term follow-up of the CASSIOPEIA randomised controlled phase 3 trial. Lancet Oncol.25, 1003–1014 (2024). - DOI - PMC - PubMed
- DARZALEX (daratumumab) injection (package insert) (Janssen Biotech, Inc., 2024).
- European Medicines Agency. DARZALEX 20 mg/mL concentrate for solution for infusion (summary of product characteristics) (Janssen Biologics BV, 2024); www.ema.europa.eu/en/documents/product-information/darzalex-epar-product...
- Facon, T. et al. Final survival analysis of daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in transplant-ineligible patients with newly diagnosed multiple myeloma: MAIA study. Paper presented European Hematology Association (EHA) Hybrid Congress (2024).
- Durie, B. G. et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet389, 519–527 (2017). - DOI - PMC - PubMed
- Durie, B. G. M. et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J.10, 53 (2020). - DOI - PMC - PubMed
- Kumar, S. K. et al. Daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) alone in transplant-ineligible patients with newly diagnosed multiple myeloma (NDMM): updated analysis of the phase 3 MAIA study. Presented at the 64th American Society of Hematology (ASH) Annual Meeting and Exposition (2022).
Gerelateerde artikelen
- Daratumumab en hyaluronidase-fihj samen met bortezomib, lenalidomide en dexamethason geeft uitstekende resultaten bij patiënten met nieuwe diagnose van multiple myeloma die niet in aanmerking komen voor stamceltransplantatie.
- Daratumumab toegevoegd aan een (VCd)-kuur (bortezomib, cyclofosfamide en dexamethason) geeft betere effectiviteit en meer patiënten bereikten een duurzame gedeeltelijke remissie in vergelijking met alleen VCd-kuur bij patienten met light chain amyloidosis
- Daratumumab (Darzalex) in combinatie met bortezomib (Velcade) en dexamethason voor eerder behandelde Multiple myeloma - Kahler vermindert kans op recidief op 1 jaar met 61 procent
- Daratumumab alleen gegeven geeft uitstekende resultaten bij patienten met actieve sluimerende Multiple Myeloma in vergelijking met actief monitoren








Plaats een reactie ...
Reageer op "Daratumumab en hyaluronidase-fihj samen met bortezomib, lenalidomide en dexamethason geeft uitstekende resultaten bij patiënten met nieuwe diagnose van multiple myeloma die niet in aanmerking komen voor stamceltransplantatie."