Onderzoekers van de Duke Universiteit, Harvard Universiteit en de Universiteit van Otago in Nieuw-Zeeland hebben de DunedinPACNI een software tool, een hulpmiddel, ontwikkeld waarmee op basis van één hersenscan - MRI tientallen jaren vantevoren kan voorspellen wie snel zal verouderen en wie langzaam zal verouderen. De DunedinPACNI voorspelt met name wie en ongeveer op welke leeftijd iemand de ziekte van Alzheimer - Dementie zal krijgen en daaraan zal overlijden.
De DunedinPACNI is ontwikkeld op basis van één hersenscan - MRI die bij 850 deelnemers aan de Dunedin studie op 45-jarige leeftijd werd genomen. De DunedinPACNI kan tekenen van toekomstige ziekten zoals de ziekte van Alzheimer - dementie opsporen tientallen jaren voordat de symptomen zich openbaren. Mensen die sneller verouderen, hadden een zwakker geheugen, meer gezondheidsproblemen en zelfs een hoger risico op vroegtijdig overlijden.
De onderzoekers ontdekten ook dat mensen met een DunedinPACNI-score die aangaf dat ze sneller verouderen, vaker te maken hadden met een afnemende algehele gezondheid, niet alleen wat betreft hun hersenfunctie. Uit datasets bleek dat mensen die volgens deze maatstaf sneller verouderden, slechter presteerden op cognitieve tests en een snellere krimp vertoonden in de hippocampus, een hersengebied dat cruciaal is voor het geheugen.
Mensen met een snellere verouderingsscore waren kwetsbaarder en hadden meer kans op leeftijdsgerelateerde gezondheidsproblemen zoals hartaanvallen, longziekten of beroertes.
De snelst verouderende mensen hadden 18% meer kans om binnen enkele jaren de diagnose van een chronische ziekte te krijgen, vergeleken met mensen met een gemiddelde verouderingssnelheid.
Nog alarmerender was dat ze binnen die tijdspanne ook 40% meer kans hadden om te overlijden dan degenen die langzamer verouderden, ontdekten de onderzoekers.
"Het verband tussen de veroudering van de hersenen en het lichaam is behoorlijk overtuigend", aldus een van de onderzoeksleiders prof. MD Ahmed Hariri. "De correlaties tussen verouderingssnelheid en dementie waren net zo sterk in andere demografische en sociaaleconomische groepen dan die waarop het model was getraind, waaronder een steekproef van mensen uit Latijns-Amerika, evenals deelnemers uit het Verenigd Koninkrijk met een laag inkomen of een niet-blanke huidskleur."
Het mooie is wel dat wanneer de DunedinPACNI-score eern slechte prognose geeft direct zou kunnen worden gestart met manieren om de aankomende de ziekte van Alzheimer - dementie te remmen, waarbij vooral ook een gezonde leefstijl en gezond voedingspatroon een grote rol zouden kunnen spelen. Zegt ook prof. MD Ahmed Hariri.
Onderstaande grafiek is gekopieerd uit het studieverslag:
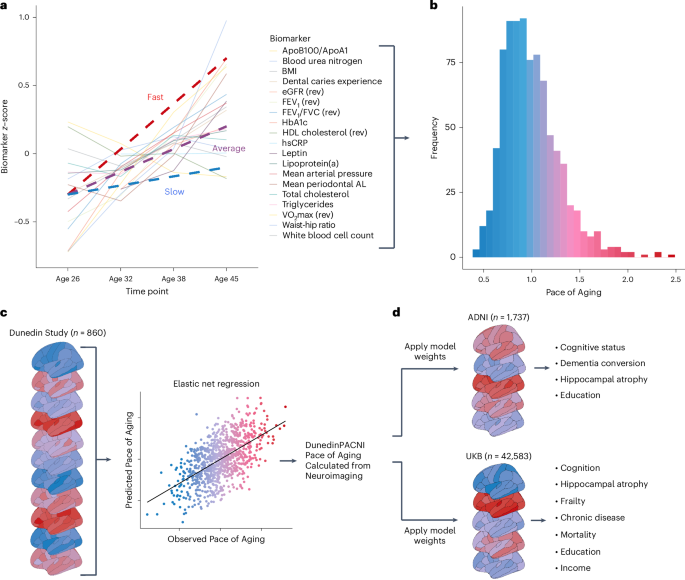
a, Plot of mean scores for all 19 biomarkers comprising the Pace of Aging across four waves of observation at ages 26, 32, 38 and 45 years in the Dunedin Study. Hypothetical individual trajectories are shown for people with relatively slow, average and fast Pace of Aging from ages 26 to 45 years. b, Distribution of Pace of Aging scores in Dunedin Study members at age 45. Warmer colors represent a faster Pace of Aging; cooler colors represent a slower Pace of Aging. c, A single T1-weighted MRI scan collected from 860 Dunedin Study members at age 45 years was used to train an elastic net regression model to predict the Pace of Aging. We call the resulting measure DunedinPACNI. d, Regression weights from the DunedinPACNI model developed in the Dunedin Study were applied to T1-weighted MRI scans collected in the ADNI and UKB datasets to derive DunedinPACNI scores. Those scores were then related to aging-related phenotypes. AL, attachment loss; Apo, apolipoprotein; BMI, body mass index; FEV1, forced expiratory volume in 1 s; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; hsCRP, high sensitivity C-reactive protein; VO2max, maximal oxygen uptake.
Het volledige studierapport is gratis in te zien of te downloaden. Klik daarvoor op de titel van het studieverslag:
- Technical Report
- Open access
- Published:
DunedinPACNI estimates the longitudinal Pace of Aging from a single brain image to track health and disease
Nature Aging (2025)
Abstract
To understand how aging affects functional decline and increases disease risk, it is necessary to develop measures of how fast a person is aging. Using data from the Dunedin Study, we introduce an accurate and reliable measure for the rate of longitudinal aging derived from cross-sectional brain magnetic resonance imaging, that is, the Dunedin Pace of Aging Calculated from NeuroImaging (DunedinPACNI). Exporting this measure to the Alzheimer’s Disease Neuroimaging Initiative, UK Biobank and BrainLat datasets revealed that faster DunedinPACNI predicted cognitive impairment, accelerated brain atrophy and conversion to diagnosed dementia. Faster DunedinPACNI also predicted physical frailty, poor health, future chronic diseases and mortality in older adults. When compared to brain age gap, DunedinPACNI was similarly or more strongly related to clinical outcomes. DunedinPACNI is a next-generation brain magnetic resonance imaging biomarker that can help researchers explore aging effects on health outcomes and evaluate the effectiveness of antiaging strategies.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The Dunedin Study data are available via managed access. Researchers who wish to use the Dunedin Study data are invited to submit a concept paper proposing the data analysis project they wish to carry out, subject to the approval of the Dunedin Study investigators. Complete instructions on accessing the Dunedin Study data can be found at https://sites.duke.edu/moffittcaspiprojects/data-use-guidelines/. The HCP data are publicly available at www.humanconnectomeproject.org/data/. The ADNI data are publicly available at https://adni.loni.usc.edu/. Researchers can apply to access all UKB data at https://ams.ukbiobank.ac.uk/ams/. The BrainLat data are publicly available at www.synapse.org/Synapse:syn51549340/wiki/624187. Source data for Fig. 1b, Fig. 2a,b, Fig. 3a,b, Fig. 4b, Fig. 5a, Fig. 6 and Fig. 7, and Extended Data Figs. 1–3 and 5–9) are published with this article.
Code availability
The DunedinPACNI algorithm is publicly available at GitHub (https://github.com/etw11/DunedinPACNI). All scripts used in the analyses presented in this article are publicly available at GitHub (https://github.com/etw11/WhitmanElliott_2024).
Acknowledgements
This research received support from the National Institute on Aging (grant no. R01AG049789 to A.R.H. and T.E.M.; grant no. R01AG032282 to A.C. and T.E.M.; grant no. R01AG073207 to A.C. and T.E.M.) and the UK Medical Research Council (grant no. MR/X021149/1 to A.C. and T.E.M.). The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council (program grant no. 16-604). We thank the Dunedin Study members, Unit research staff, the previous Study Director, Emeritus Distinguished Professor, the late R. Poulton, for his leadership during the study’s research transition from young adulthood to aging (2000–2023), and Study founder P. A. Silva. The Dunedin Unit is located within the Ngāi Tahu tribal area who we acknowledge as first peoples, tangata whenua (people of this land). This research has been conducted using the UKB Resource under application no. 67237. Data collection and sharing for ADNI is funded by the National Institute on Aging (National Institutes of Health grant no. U19 AG024904). The grantee organization is the Northern California Institute for Research and Education. In the past, ADNI has also received funding from the National Institute of Biomedical Imaging and Bioengineering, the Canadian Institutes of Health Research and private sector contributions through the Foundation for the NIH, including generous contributions from the following: AbbVie, Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation, Araclon Biotech, BioClinica, Biogen, Bristol Myers Squibb, CereSpir, Cogstate, Eisai, Elan Pharmaceuticals, Eli Lilly and Company, EuroImmun, F. Hoffmann-La Roche and its affiliated company Genentech, Fujirebio, GE Healthcare, IXICO, Janssen Alzheimer Immunotherapy Research & Development, Johnson & Johnson Pharmaceutical Research & Development, Lumosity, Lundbeck, Merck & Co., Meso Scale Diagnostics, NeuroRx Research, Neurotrack Technologies, Novartis Pharmaceuticals Corporation, Pfizer, Piramal Imaging, Servier, Takeda Pharmaceutical Company and Transition Therapeutics. We thank BrainLat and associated investigators for sharing the BrainLat dataset.
Author information
Authors and Affiliations
Contributions
E.T.W., M.L.E., A.R.K., A.C., T.E.M. and A.R.H. designed the research. E.T.W., M.L.E., A.R.K., W.C.A., T.J.A., N.J.C., S.H., D.I., T.R.M., S.R., K.S., R.T., B.S.W., A.C., T.E.M. and A.R.H. performed the research. E.T.W., M.L.E. and A.R.K. analyzed the data. E.T.W., M.L.E., A.R.K., A.C., T.E.M. and A.R.H. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
K.S., A.C. and T.E.M. are listed as inventors of DunedinPACE, a Duke University and University of Otago invention licensed to TruDiagnostic for commercial uses; however, the DunedinPACE algorithm is open access for research purposes. The other authors declare no competing interests.
Peer review
Peer review information
Nature Aging thanks Denise C. Park and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
-
03 July 2025
In the version of the article initially published, Terrie Moffitt was incorrectly listed as an equally contributing author. This has now been corrected so that Ethan Whitman and Maxwell Elliott are listed as the two equally contributing authors in the HTML and PDF versions of the article.
References
-
Belsky, D. W. et al. Quantification of biological aging in young adults. Proc. Natl Acad. Sci. USA 112, E4104–E4110 (2015).
-
Elliott, M. L. et al. Disparities in the pace of biological aging among midlife adults of the same chronological age have implications for future frailty risk and policy. Nat. Aging 1, 295–308 (2021).
-
Kuo, P.-L. et al. Longitudinal phenotypic aging metrics in the Baltimore Longitudinal Study of Aging. Nat. Aging 2, 635–643 (2022).
-
Moqri, M. et al. Biomarkers of aging for the identification and evaluation of longevity interventions. Cell 186, 3758–3775 (2023).
-
Moqri, M. et al. Validation of biomarkers of aging. Nat. Med. 30, 360–372 (2024).
-
Justice, J. N. & Kritchevsky, S. B. Putting epigenetic biomarkers to the test for clinical trials. eLife 9, e58592 (2020).
-
Melton, L. Scientists hone tools to measure aging and rejuvenation interventions. Nat. Biotechnol. 41, 1359–1361 (2023).
-
Ubaida-Mohien, C. et al. Blood biomarkers for healthy aging. Gerontology 69, 1167–1174 (2023).
-
Horvath, S. & Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 19, 371–384 (2018).
-
Rutledge, J., Oh, H. & Wyss-Coray, T. Measuring biological age using omics data. Nat. Rev. Genet. 23, 715–727 (2022).
-
Hannum, G. et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 49, 359–367 (2013).
-
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14, R115 (2013).
-
Zhang, Q. et al. Improved precision of epigenetic clock estimates across tissues and its implication for biological ageing. Genome Med. 11, 54 (2019).
-
Raffington, L. & Belsky, D. W. Integrating DNA methylation measures of biological aging into social determinants of health research. Curr. Environ. Health Rep. 9, 196–210 (2022).
-
Lu, A. T. et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 11, 303–327 (2019).
-
Levine, M. E. et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10, 573–591 (2018).
-
Sehgal, R. et al. Systems Age: a single blood methylation test to quantify aging heterogeneity across 11 physiological systems. Preprint at bioRxiv https://doi.org/10.1101/2023.07.13.548904 (2024).
-
Moffitt, T. E. Behavioral and social research to accelerate the geroscience translation agenda. Ageing Res. Rev. 63, 101146 (2020).
-
Poulton, R., Guiney, H., Ramrakha, S. & Moffitt, T. E. The Dunedin study after half a century: reflections on the past, and course for the future. J. R. Soc. N. Z. 53, 446–465 (2023).
-
Belsky, D. W. et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife 11, e73420 (2022).
-
Whitman, E. T. et al. A blood biomarker of the pace of aging is associated with brain structure: replication across three cohorts. Neurobiol. Aging 136, 23–33 (2024).
-
Sugden, K. et al. Association of pace of aging measured by blood-based DNA methylation with age-related cognitive impairment and dementia. Neurology 99, e1402–e1413 (2022).
-
Faul, J. D. et al. Epigenetic-based age acceleration in a representative sample of older Americans: associations with aging-related morbidity and mortality. Proc. Natl Acad. Sci. USA 120, e2215840120 (2023).
-
Harris, K. M. et al. Sociodemographic and lifestyle factors and epigenetic aging in US young adults: NIMHD social epigenomics program. JAMA Netw. Open 7, e2427889 (2024).
-
Guida, J. L. et al. Associations of seven measures of biological age acceleration with frailty and all-cause mortality among adult survivors of childhood cancer in the St. Jude Lifetime Cohor. Nat. Cancer 5, 731–741 (2024).
-
Cole, J. H. et al. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. Neuroimage 163, 115–124 (2017).
-
Bashyam, V. M. et al. MRI signatures of brain age and disease over the lifespan based on a deep brain network and 14 468 individuals worldwide. Brain 143, 2312–2324 (2020).
-
Yin, C. et al. Anatomically interpretable deep learning of brain age captures domain-specific cognitive impairment. Proc. Natl Acad. Sci. USA 120, e2214634120 (2023).
-
Korbmacher, M. et al. Brain asymmetries from mid- to late life and hemispheric brain age. Nat. Commun. 15, 956 (2024).
-
Han, L. K. M. et al. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol. Psychiatry 26, 5124–5139 (2021).
-
Sluiskes, M. H. et al. Clarifying the biological and statistical assumptions of cross-sectional biological age predictors: an elaborate illustration using synthetic and real data. BMC Med. Res. Methodol. 24, 58 (2024).
-
Butler, E. R. et al. Pitfalls in brain age analyses. Hum. Brain Mapp. 42, 4092–4101 (2021).
-
Vidal-Pineiro, D. et al. Individual variations in ‘brain age’ relate to early-life factors more than to longitudinal brain change. eLife 10, e69995 (2021).
-
Biondo, F. et al. Brain-age is associated with progression to dementia in memory clinic patients. Neuroimage Clin. 36, 103175 (2022).
-
Brain and body are more intertwined than we knew. Nature 623, 223–224 (2023).
-
Fischl, B. FreeSurfer. Neuroimage 62, 774–781 (2012).
-
Hsu, C.-W., Chang, C.-C. & Lin, C.-J. A Practical Guide to Support Vector Classification (National Taiwan Univ., 2016); www.csie.ntu.edu.tw/~cjlin/papers/guide/guide.pdf
-
Belsky, D. W. et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. eLife 9, e54870 (2020).
-
Haufe, S. et al. On the interpretation of weight vectors of linear models in multivariate neuroimaging. Neuroimage 87, 96–110 (2014).
-
Vidal-Piñeiro, D. et al. Accelerated longitudinal gray/white matter contrast decline in aging in lightly myelinated cortical regions. Hum. Brain Mapp. 37, 3669–3684 (2016).
-
Grydeland, H., Walhovd, K. B., Tamnes, C. K., Westlye, L. T. & Fjell, A. M. Intracortical myelin links with performance variability across the human lifespan: results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J. Neurosci. 33, 18618–18630 (2013).
-
Storsve, A. B. et al. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J. Neurosci. 34, 8488–8498 (2014).
-
Natu, V. S. et al. Apparent thinning of human visual cortex during childhood is associated with myelination. Proc. Natl Acad. Sci. USA 116, 20750–20759 (2019).
-
Raz, N. et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb. Cortex 15, 1676–1689 (2005).
-
Salat, D. H. et al. Thinning of the cerebral cortex in aging. Cereb. Cortex 14, 721–730 (2004).
-
Planche, V. et al. Structural progression of Alzheimer’s disease over decades: the MRI staging scheme. Brain Commun. 4, fcac109 (2022).
-
Salat, D. H. et al. Age-associated alterations in cortical gray and white matter signal intensity and gray to white matter contrast. Neuroimage 48, 21–28 (2009).
-
Walhovd, K. B. et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol. Aging 26, 1261–1270 (2005).
-
Van Essen, D. C. et al. The WU-Minn Human Connectome Project: an overview. Neuroimage 80, 62–79 (2013).
-
Fortea, J. et al. APOE4 homozygozity represents a distinct genetic form of Alzheimer’s disease. Nat. Med. 30, 1284–1291 (2024).
-
Jack, C. R. Jr et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 55, 484–489 (2000).
-
Jiang, R. et al. Associations of physical frailty with health outcomes and brain structure in 483 033 middle-aged and older adults: a population-based study from the UK Biobank. Lancet Digit. Health 5, e350–e359 (2023).
-
Fried, L. P. et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–M157 (2001).
-
Idler, E. L. & Benyamini, Y. Self-rated health and mortality: a review of twenty-seven community studies. J. Health Soc. Behav. 38, 21–37 (1997).
-
Chen-Xu, J. et al. Subnational inequalities in years of life lost and associations with socioeconomic factors in pre-pandemic Europe, 2009–19: an ecological study. Lancet Public Health 9, e166–e177 (2024).
-
Balaj, M. et al. Parental education and inequalities in child mortality: a global systematic review and meta-analysis. Lancet 398, 608–620 (2021).
-
Marmot, M. G. et al. Health inequalities among British civil servants: the Whitehall II study. Lancet 337, 1387–1393 (1991).
-
Greene, A. S. et al. Brain–phenotype models fail for individuals who defy sample stereotypes. Nature 609, 109–118 (2022).
-
Baez, S., Alladi, S. & Ibanez, A. Global South research is critical for understanding brain health, ageing and dementia. Clin. Transl. Med. 13, e1486 (2023).
-
Moguilner, S. et al. Brain clocks capture diversity and disparities in aging and dementia across geographically diverse populations. Nat. Med. 30, 3646–3657 (2024).
-
Stephan, B. C. M. et al. Prediction of dementia risk in low-income and middle-income countries (the 10/66 Study): an independent external validation of existing models. Lancet Glob. Health 8, e524–e535 (2020).
-
Mills, M. C. & Rahal, C. The GWAS Diversity Monitor tracks diversity by disease in real time. Nat. Genet. 52, 242–243 (2020).
-
Prado, P. et al. The BrainLat project, a multimodal neuroimaging dataset of neurodegeneration from underrepresented backgrounds. Sci. Data 10, 889 (2023).
-
Dörfel, R. P. et al. Prediction of brain age using structural magnetic resonance imaging: a comparison of accuracy and test–retest reliability of publicly available software packages. Hum. Brain Mapp. 44, 6139–6148 (2023).
-
Belsky, D. W. et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing?. Am. J. Epidemiol. 187, 1220–1230 (2018).
-
Moqri, M. et al. A unified framework for systematic curation and evaluation of aging biomarkers. Preprint at Res. Sq. https://doi.org/10.21203/rs.3.rs-4481437/v1 (2024).
-
Brayne, C. & Moffitt, T. E. The limitations of large-scale volunteer databases to address inequalities and global challenges in health and aging. Nat. Aging 2, 775–783 (2022).
-
Wig, G. S. et al. Participant diversity is necessary to advance brain aging research. Trends Cogn. Sci. 28, 92–96 (2024).
-
Falk, E. B. et al. What is a representative brain? Neuroscience meets population science. Proc. Natl Acad. Sci. USA 110, 17615–17622 (2013).
-
Elliott, M. L. et al. Brain morphometry in older adults with and without dementia using extremely rapid structural scans. Neuroimage 276, 120173 (2023).
-
Greene, D. J. et al. Behavioral interventions for reducing head motion during MRI scans in children. Neuroimage 171, 234–245 (2018).
-
Jack, C. R. Jr, Petersen, R. C., O’Brien, P. C. & Tangalos, E. G. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology 42, 183–188 (1992).
-
Kannel, W. B., Wolf, P. A., Verter, J. & McNamara, P. M. Epidemiologic assessment of the role of blood pressure in stroke. The Framingham study. JAMA 214, 301–310 (1970).
-
Sehl, M. E., Carroll, J. E., Horvath, S. & Bower, J. E. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer 6, 23 (2020).
-
Sehgal, R. et al. DNAm aging biomarkers are responsive: insights from 51 longevity interventional studies in humans. Preprint at bioRxiv https://doi.org/10.1101/2024.10.22.619522 (2024).
-
Melzer, D., Pilling, L. C. & Ferrucci, L. The genetics of human ageing. Nat. Rev. Genet. 21, 88–101 (2020).
-
Kim, K. et al. Association of adverse childhood experiences with accelerated epigenetic aging in midlife. JAMA Netw. Open 6, e2317987 (2023).
-
Reuben, A. et al. Association of childhood lead exposure with MRI measurements of structural brain integrity in midlife. JAMA 324, 1970–1979 (2020).
-
Lee, H., Lee, M. W., Warren, J. R. & Ferrie, J. Childhood lead exposure is associated with lower cognitive functioning at older ages. Sci. Adv. 8, eabn5164 (2022).
-
Livingston, G. et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446 (2020).
-
Barzilai, N., Crandall, J. P., Kritchevsky, S. B. & Espeland, M. A. Metformin as a tool to target aging. Cell Metab. 23, 1060–1065 (2016).
-
Kraus, W. E. et al. 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. Lancet Diabetes Endocrinol. 7, 673–683 (2019).
-
Richmond-Rakerd, L. S. et al. Clustering of health, crime and social-welfare inequality in 4 million citizens from two nations. Nat. Hum. Behav. 4, 255–264 (2020).
-
Springer, B. A., Marin, R., Cyhan, T., Roberts, H. & Gill, N. W. Normative values for the unipedal stance test with eyes open and closed. J. Geriatr. Phys. Ther. 30, 8–15 (2007).
-
Bohannon, R. W., Larkin, P. A., Cook, A. C., Gear, J. & Singer, J. Decrease in timed balance test scores with aging. Phys. Ther. 64, 1067–1070 (1984).
-
Vereeck, L., Wuyts, F., Truijen, S. & Van de Heyning, P. Clinical assessment of balance: normative data, and gender and age effects. Int. J. Audiol. 47, 67–75 (2008).
-
Rasmussen, L. J. H. et al. Association of neurocognitive and physical function with gait speed in midlife. JAMA Netw. Open 2, e1913123 (2019).
-
Jones, C. J. & Rikli, R. E. Measuring functional fitness of older adults. J. Act. Aging 2002, 24–30 (2002).
-
Rikli, R. E. & Jones, C. J. Functional fitness normative scores for community-residing older adults, ages 60–94. J. Aging Phys. Act. 7, 162–181 (1999).
-
Jones, C. J., Rikli, R. E. & Beam, W. C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport 70, 113–119 (1999).
-
Mathiowetz, V. et al. Grip and pinch strength: normative data for adults. Arch. Phys. Med. Rehabil. 66, 69–74 (1985).
-
Rantanen, T. et al. Midlife hand grip strength as a predictor of old age disability. JAMA 281, 558–560 (1999).
-
Tucker-Drob, E. M., Brandmaier, A. M. & Lindenberger, U. Coupled cognitive changes in adulthood: a meta-analysis. Psychol. Bull. 145, 273–301 (2019).
-
Glasser, M. F. et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124 (2013).
-
Veitch, D. P. et al. The Alzheimer’s Disease Neuroimaging Initiative in the era of Alzheimer’s disease treatment: a review of ADNI studies from 2021 to 2022. Alzheimers Dement. 20, 652–694 (2024).
-
Jack, C. R. Jr et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J. Magn. Reson. Imaging 27, 685–691 (2008).
-
Rosen, W. G., Mohs, R. C. & Davis, K. L. A new rating scale for Alzheimer’s disease. Am. J. Psychiatry 141, 1356–1364 (1984).
-
Mohs, R. C. et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis. Assoc. Disord. 11, S13–S21 (1997).
-
Folstein, M. F., Folstein, S. E. & McHugh, P. R. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
-
Nasreddine, Z. S. et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699 (2005).
-
Pfeffer, R. I., Kurosaki, T. T., Harrah, C. H. Jr, Chance, J. M. & Filos, S. Measurement of functional activities in older adults in the community. J. Gerontol. 37, 323–329 (1982).
-
Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
-
Alfaro-Almagro, F. et al. Image processing and quality control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage 166, 400–424 (2018).
-
Reuter, M., Schmansky, N. J., Rosas, H. D. & Fischl, B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418 (2012).
-
Fischl, B. & Dale, A. M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl Acad. Sci. USA 97, 11050–11055 (2000).
-
Reuter, M. & Fischl, B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage 57, 19–21 (2011).
-
Reuter, M., Rosas, H. D. & Fischl, B. Highly accurate inverse consistent registration: a robust approach. Neuroimage 53, 1181–1196 (2010).
-
Fawns-Ritchie, C. & Deary, I. J. Reliability and validity of the UK Biobank cognitive tests. PLoS ONE 15, e0231627 (2020).
-
Williams, D. M., Jylhävä, J., Pedersen, N. L. & Hägg, S. A frailty index for UK biobank participants. J. Gerontol. A Biol. Sci. Med. Sci. 74, 582–587 (2019).
-
Chadeau-Hyam, M. et al. Education, biological ageing, all-cause and cause-specific mortality and morbidity: UK biobank cohort study. EClinicalMedicine 29–30, 100658 (2020).
-
Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
-
Alfaro-Almagro, F. et al. Confound modelling in UK Biobank brain imaging. Neuroimage 224, 117002 (2021).
-
Cole, J. H. et al. Brain age predicts mortality. Mol. Psychiatry 23, 1385–1392 (2018).
-
Shrout, P. E. & Fleiss, J. L. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 86, 420–428 (1979).
-
Beer, J. C. et al. Longitudinal ComBat: a method for harmonizing longitudinal multi-scanner imaging data. Neuroimage 220, 117129 (2020).
-
Wilkinson, L. Ggplot2: elegant graphics for data analysis by WICKHAM, H. Biometrics 67, 678–679 (2011).
Gerelateerde artikelen
- Luchtvervuiling zoals fijnstof, roet en stikstofdioxide vergroten kans op ziekte van Alzheimer - Dementie blijkt uit meta-analyse van 32 grote studies
- DunedinPACE hersenscan meet hoe snel je veroudert. Het MRI beeld geannalyseerd met de DunedinPACE voorspelt kans op ziekte van Alzheimer (dementie) maar ook andere ziektes tientallen jaren vantevoren
- Eiwit TDP-43 lijkt belangrijke rol te spelen bij preventie van Frontotemporale dementie en ALS (amyotrofische laterale sclerose) , aldus het VIB-KU Leuven Centrum voor Hersenonderzoek
- Bloedtest APS2 zou de vroege en nauwkeurige diagnose van de ziekte van Alzheimer in zowel de eerste als de tweede lijn aanzienlijk kunnen verbeteren.
- Minder buikvet en meer spiermassa blijkt minder risico te geven op ontwikkelen van dementie - Alzheimer en ziekte van Parkinson
- Alzheimer - dementie is negen jaar voordat symptomen zich uiten te voorspellen, aldus nieuwe studiepublicatie
- Alzheimer - dementie is via een bloedtest jaren voordat de ziekte zich openbaart op te sporen en daardoor wellicht ook te voorkomen of uit te stellen voordat de ziekte ernstig wordt.



Plaats een reactie ...
Reageer op "DunedinPACE hersenscan meet hoe snel je veroudert. Het MRI beeld geannalyseerd met de DunedinPACE voorspelt kans op ziekte van Alzheimer (dementie) maar ook andere ziektes tientallen jaren vantevoren"