Aan dit artikel is vele uren gewerkt. Opzoeken, vertalen, op de website plaatsen enz. Als u ons wilt ondersteunen dan kan dat via een al of niet anonieme donatie. Elk bedrag is welkom hoe klein ook. Klik hier als u ons wilt helpen kanker-actueel online te houden Wij zijn een ANBI organisatie en dus is uw donatie in principe aftrekbaar voor de belasting.
16 juli 2015: Lees ook dit artikel:
28 oktober 2014: Bron: JCO October 20, 2014 JCO.2014.55.5730
Herceptin - trastuzumab toegevoegd aan chemo (Doxorubicin en Cyclophosphamide en/of Paclitaxel) post operatief, geeft 10% meer overall overlevingen op 10 jaars meting en 37% minder risico om te overlijden binnen 10 jaar bij operabele borstkanker met Her2 positieve expressie.
Dit blijkt uit de definitieve resultaten van de twee langjarige studies van de North Central Cancer Treatment Group NCCTG N9831 (Combination Chemotherapy With or Without Trastuzumab in Treating Women With HER2-Overexpressing Breast Cancer) and the National Surgical Adjuvant Breast and Bowel Project NSABP B-31 (Doxorubicin and Cyclophosphamide Plus Paclitaxel With or Without Trastuzumab in Treating Women With Node-Positive Breast Cancer That Overexpresses HER2).
De overall overleving op 10 jaar meting steeg van 75,2% naar 84%.
Median time on study was 8.4 years. Adding trastuzumab to chemotherapy led to a 37% relative improvement in OS (hazard ratio , 0.63; 95% CI, 0.54 to 0.73; P < .001) and an increase in 10-year OS rate from 75.2% to 84%. These results were accompanied by an improvement in DFS of 40% (HR, 0.60; 95% CI, 0.53 to 0.68; P < .001) and increase in 10-year DFS rate from 62.2% to 73.7%. All patient subgroups benefited from addition of this targeted anti-HER2 agent.
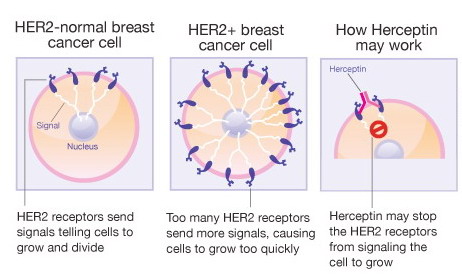
Deze studies lopen al vele vele jaren en inmiddels zijn al weer nieuwere medicijnen (zie in gerelateerde artikelen of zoek op T-DM1 - Trastuzumab Entransine of lapatinib of zoek op personalised medicine in zoekmachine rechtsboven) naast herceptin ontwikkeld en goedgekeurd voor gebruik. Maar is natuurljk een prachtig resultaat.
Ik wil er ook nog bij opmerken dat verschillende studies hebben aangetoond dat mede door de behandelingen met chemo een Her2 status kan veranderen van Her2 positief in Her2 negatief of omgekeerd. Ook blijkt soms dat uitzaaiingen in andere organen een ander karakter, een andere expressie hebben dan van de primaire borsttumor.
Het volledige studierapport: Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Planned Joint Analysis of Overall Survival From NSABP B-31 and NCCTG N9831 is tegen betaling in te zien.
Hier het abstract met de studieresultaten samengevat:
Adding trastuzumab (Herceptin) to adjuvant chemotherapy improved disease-free survival and overall survival in women with early-stage HER2-positive breast cancer.
JCO October 20, 2014 JCO.2014.55.5730
Study Details
In total, 4,046 patients with HER2-positive operable breast cancer were randomly assigned to receive doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in both trials combined. Stratification factors consisted of study, intended paclitaxel schedule (once every 3 weeks vs once per week), number of positive nodes (0–4, 4–9, ≥ 10), estrogen receptor and progesterone receptor status, and other patient and disease characteristics.
In an initial joint analysis after a median follow-up of 2 years, trastuzumab treatment was associated with a 52% reduction in disease-free survival events (P < .001) and a trend toward improved overall survival (P = .015). A second analysis at a median follow-up of 3.9 years showed that trastuzumab treatment continued to be associated with improved disease-free survival (hazard ratio = 0.52, 95% confidence interval = 0.45–0.60) and improved overall survival (HR = 0.61, 95% CI = 0.50–0.75). The current definitive overall survival analysis was scheduled to occur when a total of 710 deaths had occurred.
Improved Overall Survival and Disease-Free Survival
Median time on study was 8.4 years. Ten-year overall survival was 84% in the trastuzumab group vs 75.2% in the control group, with a hazard ratio (HR) for death of 0.63 (P < .001). On analysis adjusting for stratification factors and age, tumor size, and extent of surgery, the adjusted HR was 0.61 (P < .001). Ten-year disease-free survival was 73.7% vs 62.2% (HR = 0.60, P < .001; adjusted HR = 0.58, P < .001).
Subgroup Analyses
Similar significant overall survival benefit of trastuzumab treatment was observed in patients with estrogen receptor– and progesterone receptor–negative disease (10-year overall survival = 81.6% vs 73.0%, HR = 0.65, 95% CI = 0.53–0.80) and in those with estrogen receptor– and progesterone receptor–positive disease (10-year overall survival = 86.0% vs 77.1%, HR = 0.61, 95% CI = 0.49–0.76).
Disease-free survival benefit was also similar in patients with estrogen receptor– and progesterone receptor–negative disease (10-year disease-free survival = 70.9% vs 58.6%, HR = 0.62, 95% CI = 0.52–0.73) and those with estrogen receptor– and progesterone receptor–positive disease (10-year disease-free survival = 76.1% vs 65.1%, HR = 0.61, 95% CI = 0.51–0.72).
Subgroup analyses also showed similar overall survival benefit of trastuzumab treatment according to age < 40 years (HR = 0.67, 95% CI = 0.46–0.99), 40 to 49 years (HR = 0.65, 95% CI = 0.49–0.86), 50 to 59 years (HR = 0.68, 95% CI = 0.52–0.90), and ≥ 60 years (HR = 0.51, 95% CI = 0.37–0.69) and according to tumor size 0.1 to 2 cm (HR = 0.51, 95% CI = 0.38–0.69), 2.1 to 5 cm (HR = 0.68, 95% CI = 0.56–0.82), and > 5 cm (HR = 0.58, 95% CI = 0.39–0.88). Significant disease-free survival benefits were also observed with trastuzumab treatment in all age categories (HRs = 0.50–0.64) and tumor size categories (HRs = 0.47–0.65).
According to nodal involvement, 10-year overall survival rates for the trastuzumab vs control groups were 89.0% vs 83.1% for 0 to 3 positive nodes, 79.2% vs 70.4% for 4 to 9 positive nodes, and 71.7% vs 52.6% for ≥ 10 positive nodes.
The investigators concluded: “The addition of trastuzumab to paclitaxel after doxorubicin and cyclophosphamide in early-stage HER2-positive breast cancer results in a substantial and durable improvement in survival as a result of a sustained marked reduction in cancer recurrence.”
Edith A. Perez, MD, of the Mayo Clinic, Jacksonville, is the corresponding author for the Journal of Clinical Oncology article.
The study was supported by grants from the National Institutes of Health, NSABP, and Cancer Leukemia Group B and by Genentech.
For full disclosures of the study authors, visit jco.ascopubs.org.
- ©American Society of Clinical Oncology
Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Planned Joint Analysis of Overall Survival From NSABP B-31 and NCCTG N9831
- Edith A. Perez⇑,
- Edward H. Romond,
- Vera J. Suman,
- Jong-Hyeon Jeong,
- George Sledge,
- Charles E. Geyer Jr,
- Silvana Martino,
- Priya Rastogi,
- Julie Gralow,
- Sandra M. Swain,
- Eric P. Winer,
- Gerardo Colon-Otero,
- Nancy E. Davidson,
- Eleftherios Mamounas,
- Jo Anne Zujewski and
- Norman Wolmark
+ Author Affiliations
- Corresponding author: Edith A. Perez, MD, Mayo Clinic, 4500 San Pablo Rd, Jacksonville, FL 32224; e-mail: perez.edith@mayo.edu.
Abstract
Purpose Positive interim analysis findings from four large adjuvant trials evaluating trastuzumab in patients with early-stage human epidermal growth factor receptor 2 (HER2) –positive breast cancer were first reported in 2005. One of these reports, the joint analysis of North Central Cancer Treatment Group NCCTG N9831 (Combination Chemotherapy With or Without Trastuzumab in Treating Women With HER2-Overexpressing Breast Cancer) and the National Surgical Adjuvant Breast and Bowel Project NSABP B-31 (Doxorubicin and Cyclophosphamide Plus Paclitaxel With or Without Trastuzumab in Treating Women With Node-Positive Breast Cancer That Overexpresses HER2), was updated in 2011. We now report the planned definitive overall survival (OS) results from this joint analysis along with updates on the disease-free survival (DFS) end point.
Methods In all, 4,046 patients with HER2-positive operable breast cancer were enrolled to receive doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in both trials. The required number of events for the definitive statistical analysis for OS (710 events) was reached in September 2012. Updated analyses of overall DFS and related subgroups were also performed.
Results Median time on study was 8.4 years. Adding trastuzumab to chemotherapy led to a 37% relative improvement in OS (hazard ratio , 0.63; 95% CI, 0.54 to 0.73; P < .001) and an increase in 10-year OS rate from 75.2% to 84%. These results were accompanied by an improvement in DFS of 40% (HR, 0.60; 95% CI, 0.53 to 0.68; P < .001) and increase in 10-year DFS rate from 62.2% to 73.7%. All patient subgroups benefited from addition of this targeted anti-HER2 agent.
Conclusion The addition of trastuzumab to paclitaxel after doxorubicin and cyclophosphamide in early-stage HER2-positive breast cancer results in a substantial and durable improvement in survival as a result of a sustained marked reduction in cancer recurrence.
Gerelateerde artikelen
- trastuzumab-deruxtecan geeft uitstekende resultaten bij patienten met eerder trastuzumab en pertuzumab voorbehandelde gevorderde borstkanker met hersenuitzaaiingen
- Trastuzumab deruxtecan verbetert progressievrije overleving in vergelijking met chemotherapie na een of meer behandelingen met hormoontherapie bij patiënten met HR-positieve, HER2 lage expressie
- Trastuzumab deruxtecan geeft betere resultaten in vergelijking met de keuze van de arts bij patienten met HER2-laag, inoperabele en/of uitgezaaide borstkanker.
- Pertuzumab plus hooggedoseerde trastuzumab (herceptin) bij patiënten met HER2-positieve gemetastaseerde borstkanker en CZS uitzaaiingen met progressie na bestralingstherapie.geeft alsnog goede resultaten op stabiele ziekte en remissies
- HER2+ uitgezaaide borstkanker behandeld met trastuzumab-emtansine (T-DM1) slaat beter aan bij hoge HER2Neu expressie en geeft betere overall overleving in vergelijking met lage HER2NEU expressie.
- Immuuntherapie met trastuzumab deruxtecan geeft ook bij borstkankerpatienten met hormoongevoelige tumoren (ER pos. en HER2 lage expressie) uitstekende resultaten met zelfs 2 complete remissies met of zonder endocriene therapie
- Tucatinib in combinatie met trastuzumab en capecitabine geeft betere overall overleving bij borstkankerpatiënten met hersenmetastasen en voorkomt ontstaan van nieuwe hersenuitzaaiingen
- Immuuntherapeutisch medicijn durvalumab toegevoegd aan chemotherapie vooraf aan operatie bij patienten met triple-negatieve borstkanker geeft betere overall overleving op 3-jaars meting onafhankelijk van pathologische complete respons
- Trastuzumab deruxtecan geeft betere overall overleving na recidief ondanks trastuzumab - herceptin plus chemo in vergelijking met trastuzumab-emtansine in de behandeling van patiënten met HER2-positieve uitgezaaide borstkanker.
- Hormoonreceptor (HR)-negatieve, HER2-positieve borstkanker geeft zowel met pertuzumab en trastuzumab plus chemo met paclitaxel of zonder chemo uitstekende overlevingsresultaten
- FDA geeft goedkeuring aan tucatinib (TUKYSA), een tyrosine kinase remmer samen met trastuzumab and capecitabine voor borstkankerpatiënten met HER2 pos. mutatie die eerder een of meer eerdere behandelingen voor gevorderde borstkanker hebben gehad
- Eerst operatie van borst voor HER2 positieve borstkanker stadium IV gevolgd door systemische behandelingen geeft 44 procent meer overall overlevingen dan systemische behandelingen zonder operatie copy 1
- Trastuzumab/Emtansine geeft nog betere resultaten dan trastuzumab (Herceptin) (88 vs 77 procent ziektevrij op 3-jaars meting) bij resterend tumorweefsel in lymfklieren na chemo en operatie bij HER@ pos. borstkanker
- T-DM1 (Kadcyla) verbetert overall overleving met 27 procent versus capecitabine plus lapatinib (Tykerb) bij patienten met HER2-positieve gevorderde borstkanker met herceptin resistentie
- Herceptin plus lapatinib doet borstkankertumoren Her2 pos. verdwijnen als sneeuw voor de zon bij borstkankerpatienten met beginnende borstkanker.
- T-DM1 - (Kadcyla) preoperatief gevolgd door operatie plus herceptin, maar zonder chemo, geeft 25 procent meer - 40% vs 15 % - complete remissies bij borstkanker met Her2 pos, ongeacht hormoonstatus in vergelijking met hormoontherapie plus herceptin
- T-DM1 verdubbelt progressievrije ziekte (3 vs 6 maanden) en geeft betere overleving voor uitbehandelde borstkankerpatiënten met Her2 pos.versus behandelingskeuze van arts
- T-DM1 - Trastuzumab Emtansine verbetert significant progressie vrije overleving bij vrouwen met uitgezaaide borstkanker - HER2 positief en eerder behandeld met chemo met herceptin. in vergelijking met Capecitabine - Xeloda en lapatinib - Tykerb
- herceptin - trastuzumab geeft bij beginnende borstkanker met kleine tumoren en 1 positieve lymfklier significant betere ziektevrije tijd en overall overleving ongeacht ER en HER2 status op 8 jaars meting
- Herceptin - trastuzumab naast chemo geeft 37 procent minder kans op overlijden en 10 procent meer overlevingen op 10 jaar bij Her2 positieve borstkanker.
- Vaccin GP2 naast herceptin geeft significant betere resultaten na 3 jaar dan alleen herceptin bij Her2 positieve borstkanker copy 1
- Herceptin - Trastuzumab vergroot kansen op uitzaaiingen in het centrale zenuwstelsel bij vrouwen met HER2-Neu positieve borstkanker
- Herceptin - trastuzumab samen met Lapatinib - pertuzumab - dubbele blokkade - voor HER2-positieve borstkanker is twee keer zo effectief dan genoemde medicijnen alleen
- Een HER-2 - neu vaccin (E-75) samen met Herceptin - Trastuzumab vermindert de kans op een recidief van borstkanker met 57 procent t.o.v. een behandeling met Herceptin - Trastuzumab alleen
- FISH - Fluorescence In Situ Hybridization - vaststellen van Her2-Neu expressie via lichtgevende technieken buiten de laboratoria is superieur aan vaststellen van Her2-Neu expressie bij borstkankerpatiënten aan standaard laboratorium diagnose technieken
- Waarschuwing in the Lancet voor euforie over Herceptin. na toch uitstekende resultaten uit een fase III studie met Herceptin en borstkankerpatiënten.
- Olijfolie als aanvulling op Herceptin bevordert effect van Herceptin bij Her-2/neu borstkanker en onderdrukt het Her-2/neu (erbB-2) blijkt uit laboratorium studie.
- Herceptin - Trastuzumab naast niet op anthracycline gebaseerde chemo bij borstkanker geeft ook zelfde positieve effect op ziektevrije tijd en overall overleving, maar minder risico op hartfalen en andere bijwerkingen dan met anthracycline chemo
- Herceptin - trastuzumab geeft significant minder kans op recidief naast verschillende chemokuren bij borstkanker stadium I, II en III.
- Alvleesklierkanker: Laboratorium studies en dierstudies geven goed effect van Herceptin in combinatie met gemcitabine en/of docetaxel bij inoperabele alvleesklierkanker
- Twee nieuwe grote studies bewijzen een groot positief effect (50% minder recidieven na twee jaar) van 1 jaar Herceptin bij borstkanker met HER2-Neu expressie
- Herceptin - Trastuzumab in combinatie met anthracycline/docetaxel en of docetaxel - carboplatin geeft significant betere resultaten in ziektevrije periode bij borstkankerpatiënten met Her2-Neu positieve expressie, aldus fase III studie
- Herceptin in combinatie met verschillende chemokuren geeft significant beter effect bij borstkankerpatiënten in gerandomiseerde studie en bevestigt eerdere opvallende resultaten
- Wetenschappelijk artikel over rol en functie van Herceptin bij borstkanker. uit het Reformatorisch Dagblad..
- Ziektekostenverzekeraar Mengis gaat samen met regionale patiëntenverenigingen onderzoek doen waarom Herceptin zo weinig voorgeschreven wordt. Financiering daarvan mag geen beletsel zijn aldus Mengis in Nederlands Tijdschrift voor Geneeskunde
- Herceptin - Trastuzumab bij borstkanker: een overzicht van recente ontwikkelingen en belangrijke artikelen en studies



Plaats een reactie ...
Reageer op "Herceptin - trastuzumab naast chemo geeft 37 procent minder kans op overlijden en 10 procent meer overlevingen op 10 jaar bij Her2 positieve borstkanker."