Uit nieuwe studiegegevens blijkt dat bij patiënten met alvleesklierkanker na een operatie een combinatiebehandeling van op maat gemaakte neoantigeen-mRNA-vaccins in deze studie was dat het autogeen cevumeran, in combinatie met atezolizumab, een anti-PD-L1 medicijn en chemotherapie met mFOLFIRINOX een opvallend langdurige aanwezigheid van neoantigeen-specifieke CD8+ T-celklonen laat zien. De T-cellen bleven zeker tot 3 jaar aanwezig in de patiënten maar waarschijnlijk nagenoeg levenslang aldus de onderzoekers. Wat resulteert ook in de laatste follow-up analyse na 3,2 jaar van een veel langere recidiefvrije overleving in vergelijking met de patiënten die dezelfde behandeling kregen maar zonder toevoeging van de neoantigeen-mRNA-vaccins.
Hier de vertaling van het abstract en de belangrijkste conclusie met hulp van google translate:
- Een fundamentele uitdaging voor kankervaccins is het genereren van langlevende functionele T-cellen die specifiek zijn voor tumorantigenen. Hieruit concluderen we dat mRNA-lipoplexvaccins tegen somatische mutatie-afgeleide neoantigenen deze uitdaging kunnen oplossen bij pancreasductaal adenocarcinoom (PDAC), een dodelijke kanker met weinig mutaties.
- Bij een verlengde mediane follow-up van 3,2 jaar van een fase 1-onderzoek naar chirurgie, atezolizumab (PD-L1-remmende antistof), autogeen cevumeran1 (geïndividualiseerd neoantigeenvaccin met backbone-geoptimaliseerde uridine mRNA–lipoplex nanodeeltjes) en gemodificeerde (m) FOLFIRINOX (chemotherapie) bij patiënten met PDAC, ontdekten we dat responders met vaccin-geïnduceerde T-cellen (n = 8) een verlengde recidiefvrije overleving (RFS; mediaan niet bereikt) hebben in vergelijking met niet-responders zonder vaccin-geïnduceerde T-cellen (n = 8; mediane RFS 13,4 maanden; P = 0,007).
- Bij responders induceert autogeen cevumeran CD8+ T-celklonen met een gemiddelde geschatte levensduur van 7,7 jaar (variërend van 1,5 tot ongeveer 100 jaar), waarbij ongeveer 20% van de klonen een latente levensduur van meerdere decennia heeft die de gastheer mogelijk overleeft. Zesentachtig procent van de klonen per patiënt blijft ongeveer drie jaar na vaccinatie in aanzienlijke frequenties aanwezig, inclusief klonen met een hoge aviditeit voor PDAC-neo-epitopen.
- Met behulp van PhenoTrack, een nieuwe computationele strategie om individuele T-celfenotypes te traceren, ontdekken we dat vaccin-geïnduceerde klonen niet detecteerbaar zijn in weefsels vóór vaccinatie en tot drie jaar na vaccinatie een cytotoxische, in weefsel aanwezige geheugenachtige T-celstatus aannemen met behoud van neoantigeen-specifieke effectorfunctie.
- Twee responders vertoonden een recidief en vertoonden minder vaccin-geïnduceerde T-cellen. Bovendien werden recidiverende PDAC's verwijderd uit vaccingerichte kankerklonen.
- Zo induceert autogeen cevumeran bij PDAC de novo CD8+ T-cellen met een levensduur van meerdere jaren, een aanzienlijke omvang en duurzame effectorfuncties die PDAC-recidief kunnen vertragen. Adjuvante mRNA-lipoplex neoantigeenvaccins zouden zo een cruciaal obstakel voor kankervaccinatie kunnen oplossen.
Hier de eerdere resultaten van de studie grafisch weergegeven:
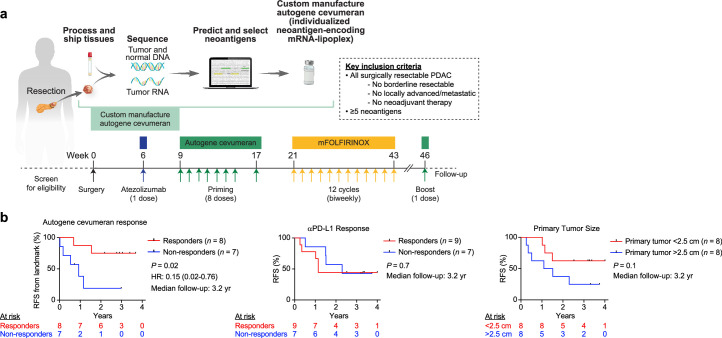
a, Trial schematic as previously reported1. Reproduced under CC BY 4.0: https://creativecommons.org/licenses/by/4.0/. b, Recurrence-free survival (RFS) from landmark time (last vaccine priming dose) stratified by autogene cevumeran (vaccine) immunologic response determined by ex vivo IFNγ ELISpot1 (left), and RFS from surgery stratified by anti-PD-L1 (atezolizumab) response (middle) and median primary tumour size (right). HR, hazard ratio with 95% confidence interval. Black tick marks, censorship points. n is the number of individual patients. P values by two-tailed log-rank test (b).
Het volledige studierapport is gratis in te zien of te downloaden, zie daarvoor het abstract:
RNA neoantigen vaccines prime long-lived CD8+ T cells in pancreatic cancer
- PMID: 39972124
- PMCID: PMC11946889
- DOI: 10.1038/s41586-024-08508-4
Abstract
A fundamental challenge for cancer vaccines is to generate long-lived functional T cells that are specific for tumour antigens. Here we find that mRNA-lipoplex vaccines against somatic mutation-derived neoantigens may solve this challenge in pancreatic ductal adenocarcinoma (PDAC), a lethal cancer with few mutations. At an extended 3.2-year median follow-up from a phase 1 trial of surgery, atezolizumab (PD-L1 inhibitory antibody), autogene cevumeran1 (individualized neoantigen vaccine with backbone-optimized uridine mRNA-lipoplex nanoparticles) and modified (m) FOLFIRINOX (chemotherapy) in patients with PDAC, we find that responders with vaccine-induced T cells (n = 8) have prolonged recurrence-free survival (RFS; median not reached) compared with non-responders without vaccine-induced T cells (n = 8; median RFS 13.4 months; P = 0.007). In responders, autogene cevumeran induces CD8+ T cell clones with an average estimated lifespan of 7.7 years (range 1.5 to roughly 100 years), with approximately 20% of clones having latent multi-decade lifespans that may outlive hosts. Eighty-six percent of clones per patient persist at substantial frequencies approximately 3 years post-vaccination, including clones with high avidity to PDAC neoepitopes. Using PhenoTrack, a novel computational strategy to trace single T cell phenotypes, we uncover that vaccine-induced clones are undetectable in pre-vaccination tissues, and assume a cytotoxic, tissue-resident memory-like T cell state up to three years post-vaccination with preserved neoantigen-specific effector function. Two responders recurred and evidenced fewer vaccine-induced T cells. Furthermore, recurrent PDACs were pruned of vaccine-targeted cancer clones. Thus, in PDAC, autogene cevumeran induces de novo CD8+ T cells with multiyear longevity, substantial magnitude and durable effector functions that may delay PDAC recurrence. Adjuvant mRNA-lipoplex neoantigen vaccines may thus solve a pivotal obstacle for cancer vaccination.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: L.A.R., Z.M.S., B.D.G. and V.P.B. are inventors on patent applications related to work on antigen cross-reactivity and tracking vaccine-induced T cell clones. B.D.G. and V.P.B. are inventors on a patent application on neoantigen quality modelling. L.A.R. is an inventor of a patent related to oncolytic viral therapy. B.D.G. has received honoraria for speaking engagements from Merck, Bristol Meyers Squibb and Chugai Pharmaceuticals; has received research funding from Bristol Meyers Squibb, Merck and ROME Therapeutics; and has been a compensated consultant for Darwin Health, Merck, PMV Pharma, Shennon Biotechnologies, Synteny and Rome Therapeutics of which he is a co-founder. V.P.B. reports honoraria and research support from Genentech and research support from Bristol-Myers Squibb. A.S.E received royalties from Up-To-Date. A.V. reports research funding from Lilly, Verastem, BioMed Valley Discoveries, Bristol-Myers Squibb and Silenseed. A.C.W. reports the following: Histosonics, consulting and Ipsen, clinical trial funding. E.M.O. reports research funding to the institution from: Genentech/Roche, BioNTech, AstraZeneca, Arcus, Elicio, Parker Institute, NIH/NCI, Digestive Care and Break Through Cancer; consulting via Data and Safety Monitoring Board (DSMB) for: Arcus, Alligator, Agenus, BioNTech, Ipsen, Merck, Moma Therapeutics, Novartis, Syros, Leap Therapeutics, Astellas, BMS, Fibrogen, Revolution Medicine, Merus Agios (spouse), Genentech-Roche (spouse), Eisai (spouse) and Servier (Spouse). J.D. owns stock in Alnylam Pharmaceuticals, Arrowroot Acquisition and Ionis Pharmaceuticals. T.M. is a co-founder and holds equity in IMVAQ Therapeutics; is a consultant for Immunos Therapeutics, ImmunoGenesis and Pfizer; has research support from Bristol-Myers Squibb, Surface Oncology, Kyn Therapeutics, Infinity Pharmaceuticals, Peregrine Pharmaceuticals, Adaptive Biotechnologies, Leap Therapeutics and Aprea; and has patents on applications related to work on oncolytic viral therapy, alphavirus-based vaccine, neoantigen modelling, CD40, GITR, OX40, PD-1 and CTLA-4. J.D.W. is a consultant for Apricity, CellCarta, Ascentage Pharma, AstraZeneca, Bicara Therapeutics, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Dragonfly, Georgiamune, Imvaq, Larkspur, Psioxus, Recepta, Tizona and Sellas. J.D.W. receives grant and research support from Bristol-Myers Squibb and Sephora. J.D.W. has equity in Apricity, Arsenal IO, Ascentage, Imvaq, Linneaus, Georgiamune, Maverick and Tizona Therapeutics. W.P. reports research funding to institution from: Merck, Astellas, Miracogen and Amgen; consultancy or advisory board activity for: Astellas and EXACT Therapeutics; honoraria for Continuing Medical Education (CME) from: American Physician Institute and Integrity. O.T. and U.S. are co-founders, management board members and employees at BioNTech. E.D., L.M. and F.M. are employees at BioNTech. I.R., M.Y. and I.M. are employees at Genentech. The other authors declare no competing interests.
Similar articles
-
Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer.Nature. 2023 Jun;618(7963):144-150. doi: 10.1038/s41586-023-06063-y. Epub 2023 May 10.PMID: 37165196 Free PMC article. Clinical Trial.
-
Autogene cevumeran with or without atezolizumab in advanced solid tumors: a phase 1 trial.Nat Med. 2025 Jan;31(1):152-164. doi: 10.1038/s41591-024-03334-7. Epub 2025 Jan 6.PMID: 39762422 Free PMC article. Clinical Trial.
-
CD137 agonist-based combination immunotherapy enhances activated, effector memory T cells and prolongs survival in pancreatic adenocarcinoma.Cancer Lett. 2021 Feb 28;499:99-108. doi: 10.1016/j.canlet.2020.11.041. Epub 2020 Nov 30.PMID: 33271264 Free PMC article.
-
Neoantigen-based immunotherapy in pancreatic ductal adenocarcinoma (PDAC).Cancer Lett. 2020 Oct 10;490:12-19. doi: 10.1016/j.canlet.2020.06.011. Epub 2020 Jun 23.PMID: 32590021 Review.
-
Advances in Vaccine-Based Therapies for Pancreatic Cancer.J Gastrointest Cancer. 2025 Feb 12;56(1):62. doi: 10.1007/s12029-025-01165-4.PMID: 39939414 Free PMC article. Review.
Cited by
-
Advances in mRNA vaccine therapy for breast cancer research.Discov Oncol. 2025 May 6;16(1):673. doi: 10.1007/s12672-025-02542-y.PMID: 40327249 Free PMC article. Review.
-
Vaccine therapies for glioma: clinical frontiers and potential breakthrough.Front Oncol. 2025 Jun 25;15:1613332. doi: 10.3389/fonc.2025.1613332. eCollection 2025.PMID: 40661781 Free PMC article. Review.
-
The Application of mRNA Technology for Vaccine Production-Current State of Knowledge.Vaccines (Basel). 2025 Apr 4;13(4):389. doi: 10.3390/vaccines13040389.PMID: 40333251 Free PMC article. Review.
-
Improving outcomes of patients with pancreatic cancer.Nat Rev Clin Oncol. 2025 Jun;22(6):439-456. doi: 10.1038/s41571-025-01019-9. Epub 2025 May 6.PMID: 40329051 Review.
-
Cancelling mRNA studies is the highest irresponsibility.Nature. 2025 Aug;644(8077):579. doi: 10.1038/d41586-025-02612-9.PMID: 40817197 No abstract available.
References
- 1.Rojas, L. A. et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature618, 144–150 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neoptolemos, J. P. et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA304, 1073–1081 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Uesaka, K. et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet388, 248–257 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos, J. P. et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet389, 1011–1024 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Murugan, A., Mora, T., Walczak, A. M. & Callan, C. G. Statistical inference of the generation probability of T-cell receptors from sequence repertoires. Proc. Natl Acad. Sci. USA109, 16161–16166 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glanville, J. et al. Identifying specificity groups in the T cell receptor repertoire. Nature286, 958 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sallusto, F., Lanzavecchia, A., Araki, K. & Ahmed, R. From vaccines to memory and back. Immunity33, 451–463 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebhardt, T., Park, S. L. & Parish, I. A. Stem-like exhausted and memory CD8+ T cells in cancer. Nat. Rev. Cancer23, 780–798 (2023). [DOI] [PubMed] [Google Scholar]
- 9.Buggert, M., Price, D. A., Mackay, L. K. & Betts, M. R. Human circulating and tissue-resident memory CD8+ T cells. Nat. Immunol.24, 1076–1086 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Fonseca, R. et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat. Immunol.21, 412–421 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park, S. L. et al. Tissue-resident memory CD8+ T cells promote melanoma–immune equilibrium in skin. Nature565, 366–371 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Mackay, L. K. et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science352, 459–463 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Milner, J. J. et al. Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature552, 253–257 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson, C. M. et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature442, 299–302 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Crowl, J. T. et al. Tissue-resident memory CD8+ T cells possess unique transcriptional, epigenetic and functional adaptations to different tissue environments. Nat. Immunol.23, 1121–1131 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kragten, N. A. M. et al. Blimp‐1 induces and Hobit maintains the cytotoxic mediator granzyme B in CD8 T cells. Eur. J. Immunol.48, 1644–1662 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Chen, J. et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature567, 530–534 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheeler, B. D. et al. The lncRNA Malat1 inhibits miR-15/16 to enhance cytotoxic T cell activation and memory cell formation. eLife12, RP87900 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakim, L. M., Gupta, N., Mintern, J. D. & Villadangos, J. A. Enhanced survival of lung tissue-resident memory CD8+ T cells during infection with influenza virus due to selective expression of IFITM3. Nat. Immunol.14, 238–245 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Ostkamp, P. et al. A single-cell analysis framework allows for characterization of CSF leukocytes and their tissue of origin in multiple sclerosis. Sci. Transl. Med.14, eadc9778 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Giles, J. R., Globig, A.-M., Kaech, S. M. & Wherry, E. J. CD8+ T cells in the cancer-immunity cycle. Immunity56, 2231–2253 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. Ca. Cancer J. Clin.73, 17–48 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Akondy, R. S. et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature552, 362–367 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marraco, S. A. F. et al. Long-lasting stem cell–like memory CD8+ T cells with a naïve-like profile upon yellow fever vaccination. Sci. Transl. Med.7, 282ra48 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Balachandran, V. P. et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature551, 512–516 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature534, 396–401 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Hu, Z. et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat. Med.27, 515–525 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Łuksza, M. et al. Neoantigen quality predicts immunoediting in survivors of pancreatic cancer. Nature606, 389–395 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahin, U. et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature595, 572–577 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature547, 222–226 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Holtkamp, S. et al. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood108, 4009–4017 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Kreiter, S. et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature520, 692–696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robins, H. et al. Ultra-sensitive detection of rare T cell clones. J Immunol Methods375, 14–19 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlson, C. S. et al. Using synthetic templates to design an unbiased multiplex PCR assay. Nat. Commun.4, 2680 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Huang, H., Wang, C., Rubelt, F., Scriba, T. J. & Davis, M. M. Analyzing the Mycobacterium tuberculosis immune response by T-cell receptor clustering with GLIPH2 and genome-wide antigen screening. Nat. Biotechnol.38, 1194–1202 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol.19, 15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ceglia, N. et al. TCRi: Information theoretic metrics for single cell RNA and TCR sequencing in cancer. Preprint at bioRxiv10.1101/2022.10.01.510457 (2022).
- 38.Ceglia, N. et al. Identification of transcriptional programs using dense vector representations defined by mutual information with GeneVector. Nat. Commun.14, 4400 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedregosa, F. et al. Scikit-learn: machine learning in Python. Preprint at arXiv10.48550/arxiv.1201.0490 (2012).
- 40.Sethna, Z., Elhanati, Y., Callan, C. G., Walczak, A. M. & Mora, T. OLGA: fast computation of generation probabilities of B- and T-cell receptor amino acid sequences and motifs. Bioinformatics35, btz035- (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcou, Q., Mora, T. & Walczak, A. M. High-throughput immune repertoire analysis with IGoR. Nat. Commun.9, 561 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Travaglini, K. J. et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature587, 619–625 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at arXiv10.48550/arxiv.1303.3997 (2013).
- 44.McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res.20, 1297–1303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cibulskis, K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat. Biotechnol.31, 213–219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saunders, C. T. et al. Strelka: accurate somatic small-variant calling from sequenced tumor–normal sample pairs. Bioinformatics28, 1811–1817 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Deshwar, A. G. et al. PhyloWGS: reconstructing subclonal composition and evolution from whole-genome sequencing of tumors. Genome Biol.16, 35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerelateerde artikelen
- ESMO 2024: aanbevolen abstracten door vooraanstaande oncologen en medisch specialisten gerelateerd aan studies bij spijsverteringskanker waaronder vormen van darmkanker, alvleesklierkanker, slokdarmkanker, leverkanker en maagkanker copy 1
- ASCO 2024: abstracten van ASCO 2024 gerelateerd aan alvleesklierkanker en galwegenkanker copy 1
- Alvleesklierkanker: Overzicht van recente studiepublicaties voor behandelen van alvleesklierkanker in verschillende stadia
- Alle alvleesklierkankerpatienten komen in 1 grote landelijke wetenschappelijke studie. Het Deltaplan alvleesklierkanker moet de grote sterfte aan alvleesklierkanker stoppen
- Studiepublicaties van voeding, voedingstoffen, niet-toxische middelen en behandelingen uit literatuurlijst van arts-bioloog drs. Engelbert Valstar, specifiek bij alvleesklierkanker copy 1
- Bestraling vooraf aan operatie bij alvleesklierkanker zou significant langere overlevingstijd en overall overleving geven, aldus nieuwe studie. Maar bestraling vooraf blijft controversieel, aldus onderzoekers.
- Cyberknife: Alvleesklierkankerpatienten kunnen ook behandeld worden met cyberknife in Nederland
- Chemo: een overzicht van recente ontwikkelingen en belangrijke studies over chemo en radiotherapie - bestraling
- Daraxonrasib (RMC-6236) geeft bij patienten met gevorderde voorbehandelde alvleesklierkanker met KRAS G12X mutaties uitstekende resultaten op mediane overleving
- Dendritische celtherapie met allogeen tumorlysaat (MesoPher) in combinatie met een CD40-agonist (Mitazalimab) geeft uitstekende resultaten bij gevorderde alvleesklierkanker aldus prof. dr. Casper van Eyck en nieuwe studie is geopend.
- Diagnose van alvleesklierkanker, een overzicht
- HIFU - High Intensity Focused Ultrasound blijkt een effectieve behandeling voor alvleesklierkanker. HIFU gebruikt gerichte hitte om kankercellen aan te pakken en te vernietigen, en er is minimale schade aan het lichaam. copy 1
- Hyperthermie: BSD opent fase III studie voor patienten met alvleesklierkanker. Studie wordt uitgevoerd met chemo - cisplatin met gemcitabine en alleen met gemcitabine copy 1
- Immuuntherapie bij alvleesklierkanker: een overzicht
- Jodium-125 implantatie - inwendige bestraling - is veel minder belastend voor de patiënt met operabele alvleesklierkanker en geeft dezelfde resultaten op overall overleving en veel betere kwaliteit van leven in vergelijking met Whipple operatie
- Nanoknife - irreversible electroporation verbetert ziektevrije tijd en mediane overall overleving bij inoperable alvleesklierkanker blijkt uit PANFIRE studie
- Neoantigeen-mRNA-vaccins in combinatie met immuuntherapie met anti-PD-L1 medicijnen en chemotherapie na operatie geeft spectaculaire recidiefvrije overleving bij operabele alvleesklierkanker met KRAS mutaties
- Operatie: De Dutch Pancreatic Cancer Audit (DPCA) een monitoringsysteem dat bepaalt hoe en wanneer een operatie van alvleesklierkanker kan worden uitgevoerd geeft uitstekende resultaten
- Overzicht van studies met medicijnen en behandelingen om tumoren met KRAS mutaties aan te pakken. Vooral combinatiebehandelingen zijn veelbelovend. copy 1
- PDT - Fotodynamische therapie beloftevol bij inoperabele alvleesklierkanker. blijkt uit verschillende studies.
- Pemigatinib, een FGFR remmer, voor patiënten met eerder behandelde galwegenkanker geeft bij patienten met een FGFR2-mutatie uitstekende resultaten met een objectieve respons van 35 procent.versus 0 procent bij wie geen FGFR2 mutatie had copy 1
- Personalised medicine: Operabele alvleesklierkankerpatienten met overexpressie van Her2-Neu hebben significant kortere overlevingstijd dan alvleesklierkankerpatienten met normale Her2-Neu expressiewaarden.
- Tumor Treating Fields naast gemcitabine en nab-paclitaxel geeft bij patienten met inoperabele lokaal uitgezaaide alvleeskanker langere overleving en langere overleving zonder pijn in vergelijking met alleen gemcitabine en nab-paclitaxel
- von Hippel–Lindau ziekte blijkt uitstekend te behandelen te zijn met Belzutifan en voorkomt verder ontwikkelen van neuro endocriene tumoren copy 1
- Regulier - alvleesklierkanker - pancreaskanker: actuele ontwikkelingen en belangrijke studies binnen de reguliere oncologie: een overzicht








Plaats een reactie ...
Reageer op "Neoantigeen-mRNA-vaccins in combinatie met immuuntherapie met anti-PD-L1 medicijnen en chemotherapie na operatie geeft spectaculaire recidiefvrije overleving bij operabele alvleesklierkanker met KRAS mutaties"