Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
29 april 2017: Bron: FDA - Food and Drug Administration en J Cancer Res Clin Oncol. 2017 Feb;143(2):337-345.
De FDA geeft toestemming voor gebruik van Midostaurin (Merknaam Rydapt) naast chemo bij volwassen patiënten met een nieuwe diagnose van AML - Acute Myeloide Leukemie welke een FLT3 mutatie hebben . Al eerder gaven zij toestemming voor gebruik van midostaurin bij gevorderde AML - Actue Myeloide Leukemie.
De FDA geeft de toestemming op basis van verschillende fase III studies die allemaal, ook bij AML - Acute Myeloide Leukemie met geen FLT3 mutatie, een verbeterde levensverlenging gaven te zien. Echter bij die patiënten met een FLT3 mutatie waren de resultaten van midostaurin statistisch significant. De FLT3 mutatie wordt gemeten via de zogeheten LeukoStrat CDx FLT3 Mutation Assay (Invivoscribe Technologies).
De FLT3 mutatie wordt gevonden bij 30% tot 35% van de patiënten met AML en verhoogde FLT3 expressie wordt geassocieerd met een slechtere prognose van de levensduur. Wanneer de patienten Midostaurin (Ryadt) naast hun chemo krijgen stijgt de mediane overall overleving: 8,7 maanden versus 3 maanden. Maar bij deze patiënten kwamen ook langdurige totale remissies voor nadat zij Midostaurin gebruikten.
In dit studierapport: Midostaurin: an emerging treatment for acute myeloid leukemia patients wordt een review gegeven van de meeste studies die zijn gedaan met midostaurin de afgelopen tien jaar. Onderaan staat abstract:. Hier een overzicht van een aantal studies. (Tekst gaat onder grafiek verder):
Table 1
Published clinical trials of midostaurin: single-agent trials and combination-agent trials
| Reference | N | Study population | FLT3 | Phase | Treatment (PO) | Key results | Comments | ||
|---|---|---|---|---|---|---|---|---|---|
| Single-agent trials | |||||||||
| 16 | 32 | Refractory or unresponsive solid tumors | Not reported | I | Dose escalation (midostaurin 12.5 mg PO daily to 100 mg PO TID) | First to report Cmax, t1/2 in study subjects. Most common AEs: nausea, vomiting, fatigue, diarrhea. 225–300 mg/d considered too toxic for future study | First in human dose-escalation study Correlatives (PKC inhibition) reported separately |
||
| 17 | 30 | Advanced MDS Relapsed/refractory AML Newly diagnosed AML ineligible for induction chemotherapy |
mut (90% ITD) | IIB 2-stage | Midostaurin 75 mg PO TID | Most common AEs: nausea, vomiting 3 fatal pulmonary events 70% had reduction of >50% in peripheral blast count; 30% had >50% reduction in BM blast count 2 went onto BMT after initial response |
First trial in AML or MDS Correlative: pFLT3 was decreased in peripheral blood monocytes and bone marrow aspirate in the subset of patients tested (n=5); all responded |
||
| 23 | 95 | Relapsed/refractory AML Newly diagnosed AML ineligible for induction chemotherapy MDS (RAEB ± transformation or CML) |
WT (63%) mut (37%) (76% ITD) |
IIB | Midostaurin 50 PO BID or 100 mg PO BID |
Best response: PR in 1% HI: 46% FLT3 mut, 35% FLT3-WT BR: 71% FLT3 mut, 42% FLT3-WT Higher BR in previously untreated patients ND in median onset of BR (29 days) or TTF between FLT3-WT and mut patients BR correlated to drug (and metabolite) concentrations in plasma |
First trial to compare responses in WT versus mut FLT3 Trough concentrations of midostaurin (and metabolites) reported |
||
| Combination trials | |||||||||
| 20 | 69 | Newly diagnosed AML, age 18–60 years | WT (72%) mut (28%) |
IB | 6 dose schedules of 50–100 mg BID midostaurin with standard daunorubicin and cytarabine induction (3+7) | Most common AEs: hematologic 100 mg BID: CR in 45% (35% WT, 83% mut); 79% discontinuation rate 50 mg BID: CR in 80% (74% WT, 92% mut) ND in OS at 1 and 2 years (WT vs mut) Superior tolerance with sequential vs concomitant administration Possible pharmacokinetic interaction with daunorubicin |
First combination trial with 7+3 Defined dosing schedule for Phase III trial NCT00651261, in progress |
||
| 13 | 16 | Newly diagnosed AML, age ≥18 years, not eligible for std induction Relapsed/refractory AML, age ≥18 years |
WT (87%) mut (13%) |
I | Decitabine days 1–5 Midostaurin days 8–21 (25–50 mg PO BID) or days 1–28 (50 mg PO BID) |
Most common AEs: hematologic 2 fatal complications of viral PNA 3 DLTs (cardiac or pulmonary) CR + CRi: 25% (duration 28–331 days) No detectable pharmacokinetic interaction with decitabine |
First combination trial with decitabine Preclinical correlatives suggested synergy with decitabine (in apoptosis, FLT3 phosphorylation and downstream signaling) |
||
| 18 | 54 | Untreated and previously treated AML or high-risk MDS | WT (26%) mut (74%) (68% ITD, 6% ITD + D835Y) |
I/II | Azacitidine (IV or SC) days 1–7 Midostaurin days 8–21 (25–50 mg BID) up to 12 cycles |
Grade 3–4 hematological AEs in 100%; Grade 3–4 nonhematological toxicity in 70% BR: 84% (PB) 53% (BM) ORR 26% (33% in FLT3-ITD pts w/o prior exposure to FLT3 inhibitors), CR 2%, CRi 11%, MLFS 11%, PR 1% (74% were primary refractory) FLT3-ITD mut was not a/w ORR Median RD: 20 weeks No detectable pharmacokinetic interactions with azacitidine |
First combination trial with azacytidine Correlative: pFLT3 |
||
| 24 | 17 | Untreated AML, age ≥70 years Relapsed AML, any age | WT (100%) mut (0%) |
I | Aza (75 mg/m2) days 1–7 Midostaurin days 8–21 (25, 50, or 75 mg PO BID) |
Most common AEs: hematologic 3 G3 GI toxicities; 1 required hospitalization CR in 3 patients (12%; duration 7, 12, 12 m) RR 18% |
Only study to report patient compliance (high) | ||
Abbreviations: AE, adverse event; AML, acute myeloid leukemia; BM, bone marrow; BMT, bone marrow transplant; BR, blast response; CR, complete remission; CRi, CR with incomplete bone marrow recovery; DLT, dose limiting toxicity; FLT3, fms-like tyrosine kinase; G, grade; HI, hematologic improvement; ITD, internal tandem duplication; m, months; MDS, myelodysplastic syndrome; MLFS, morphologic leukemia free status; ORR, overall response rate; PB, peripheral blood; PNA, pneumonia; PR, partial response; TTF, time to treatment failure; WT, wild type; mut, mutation; Aza, azacitidine; PO, by mouth; PKC, protein kinase C; RAEB, refractory anemia with excess blasts; CML, chronic myeloid leukemia; ND, no difference; IV, intravenous; SC, subcutaneous; RD, response duration; a/w; associated with; GI, gastrointestinal; OS, overall survival; BID, twice daily; TID, three times daily; RR, response rate; std, standard; w/o, without.
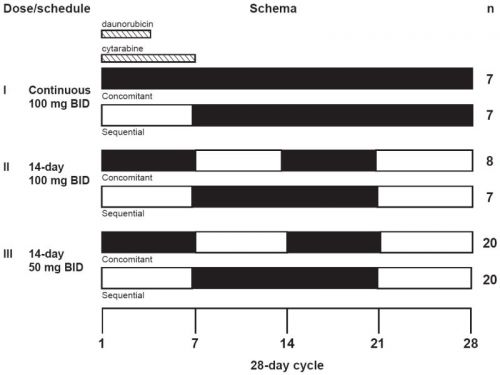
Zie deze studie opzet hoe het studieprotocol eruit zag / ziet: Daunorubicin, Cytarabine, and Midostaurin in Treating Patients With Newly Diagnosed Acute Myeloid Leukemia
Hier een grafiek van de overall overleving. Tekst gaat verder onder de grafieken
Fig 3

a. Overall survival probability in patients with FLT3–wild-type and FLT3-mutant AML treated on dose schedule III. Overall survival was assessed without censoring for alternative therapies such as stem cell transplant.
b. Disease-free survival probability in patients with FLT3–wild-type and FLT3-mutant AML treated on dose schedule III.
Open boxes represent values from patients on the concomitant arm (day 1-7 and day 15-22 dosing); closed circles represent values from patients on the sequential arm (day 8-22 dosing).
Hier een schema van de dosering zoals die is gebruikt bij de meeste studies:
Ook in deze studie al uit 2012: Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia blijkt midostautin een veel betere mediane overleving te geven bij jongere patienten met AML met veel complete remissies:
De complete remissies voor de midostaurin 50-mg 2x per dag dosering werd bereikt bij 80% (FLT3–wild-type: 20 van de 27 patiënten [74%], en met een FLT3 mutatie bij 12 van de 13 patiënten [92%]). Overall overleving (OS) voor patienten met een FLT3-mutant AML waren daarin min of meer gelijk in verschillen na 1 en 2 jaar (0.85 en 0.62, respectievelijk) aan de FLT3–wild-type deelnemers (0.78 en 0.52, respectivelijk). Midostaurin in combinatie met standaard chemotherapie toont een hoge complete remissie response en overall overlevingscijfers bij nieuw gediagnosteerde jong volwassenen met AML - Acute Myeloide Leukemie en werd algemeen genomen goed getolereerd op een dosis van 50 mg2x per dag gedurende 14 dagen.
In deze studie: Outcome of FLT3-ITD-positive acute myeloid leukemia: impact of allogeneic stem cell transplantation and tyrosine kinase inhibitor treatment. werd onderzocht wat het effect is van een FLT3 mutatie wanneer patiënten met AML een allogene beenmergtransplantatie krijgen:
CONCLUSIE:
This "real-life" data reflect the continuing challenge of FLT3-ITD-positive AML and confirm the poor outcome even after allogeneic SCT. Furthermore, efficacy of TKI treatment of relapsed or refractory FLT3-ITD AML is still limited and requires substantial improvement, e.g., by the introduction of second-generation inhibitors targeting constitutively active FLT3.
Samenvattend: Een FLT3 mutatie is een voorspeller van een slechte prognose, zelfs bij een allogene stamceltransplantatie. Medicijnen die effectief werkzaam zijn op deze FLT3 mutatie zijn dan ook noodzakelijk voor patiënten met AML - Acute Myeloide Leukemie.
Abstract van de reviewstudie: Midostaurin: an emerging treatment for acute myeloid leukemia patients welke gratis is in te zien staat hieronder met referentieliijst onderaan artikel:
Administration of midostaurin to relapsed/refractory MDS and AML patients confers a robust anti-blast response sufficient to bridge a minority of patients to transplant. In combination with histone deacetylase inhibitors, responses appear comparable to historic controls, while the addition of midostaurin to standard induction chemotherapy may prolong survival in FLT3-ITD mutant patients.
Midostaurin: an emerging treatment for acute myeloid leukemia patients
Abstract
Acute myeloid leukemia (AML) is a hematologic malignancy that carries a poor prognosis and has garnered few treatment advances in the last few decades. Mutation of the internal tandem duplication (ITD) region of fms-like tyrosine kinase (FLT3) is considered high risk for decreased response and overall survival. Midostaurin is a Type III receptor tyrosine kinase inhibitor found to inhibit FLT3 and other receptor tyrosine kinases, including platelet-derived growth factor receptors, cyclin-dependent kinase 1, src, c-kit, and vascular endothelial growth factor receptor. In preclinical studies, midostaurin exhibited broad-spectrum antitumor activity toward a wide range of tumor xenografts, as well as an FLT3-ITD-driven mouse model of myelodysplastic syndrome (MDS). Midostaurin is orally administered and generally well tolerated as a single agent; hematologic toxicity increases substantially when administered in combination with standard induction chemotherapy. Clinical trials primarily have focused on relapsed/refractory AML and MDS and included single- and combination-agent studies. Administration of midostaurin to relapsed/refractory MDS and AML patients confers a robust anti-blast response sufficient to bridge a minority of patients to transplant. In combination with histone deacetylase inhibitors, responses appear comparable to historic controls, while the addition of midostaurin to standard induction chemotherapy may prolong survival in FLT3-ITD mutant patients. The response of some wild-type (WT)-FLT3 patients to midostaurin therapy is consistent with midostaurin’s ability to inhibit WT-FLT3 in vitro, and also may reflect overexpression of WT-FLT3 in those patients and/or off-target effects such as inhibition of kinases other than FLT3. Midostaurin represents a well-tolerated, easily administered oral agent with the potential to bridge mutant and WT-FLT3 AML patients to transplant and possibly deepen response to induction chemotherapy. Ongoing studies are investigating midostaurin’s role in pretransplant induction and posttransplant consolidation therapy
Conclusion
Midostaurin’s inhibition of WT and mutant FLT3, ease of administration, and general good tolerance make it an attractive agent in the limited armamentarium of treatments for FLT3-mutant (and WT) AML. The preclinical and clinical studies published to date provide a starting point for the determination of midostaurin’s potential clinical utility in AML treatment. As a single agent, midostaurin has the potential to induce responses in relapsed/refractory AML or MDS that carry a minority of patients to a potentially curative HCT. When administered in combination with induction chemotherapy, midostaurin has the potential to deepen and/or prolong response. Whether midostaurin improves relapse-free survival after transplant remains an open question, which is under active investigation.
On the basis of the observations discussed earlier, we recommend that midostaurin be regarded as a single agent in relapsed/refractory AML or MDS patients who are HCT candidates as a potential bridge to curative therapy. This recommendation is independent of FLT3 mutation status given the documented response of FLT3-WT patients (though sometimes inferior to FLT3-mutated patients) to treatment. We also recommend enrollment in clinical trials of midostaurin for induction or consolidation therapy. Hopefully, the answer to important clinical questions such as how deeply midostaurin improves response to induction chemotherapy, if its use can extend duration of response after HCT, and how it compares to other emerging FLT3 inhibitors in efficacy and safety will be forthcoming.
References
Gerelateerde artikelen
- Gepersonaliseerde medicijnen gegeven op basis van meerdere genentesten bij patienten met Acute Myeloide Leukemie (AML) geeft veel betere overleving en ziektecontrole dan standaard behandelingen
- All Trans Retinoïnezuur (ATRA) naast lage doses azacytidine en pioglitazon geeft hoopvolle resultaten bij ≥ 60 jaar patiënten met acute myeloïde leukemie die ongevoelig is voor chemotherapie en geen stamceltransplantatie kunnen ondergaan
- Leukemie. AML - Acute Myeloide Leukemie. H. bereikt volledige remissie van zijn Acute Myeloide Leukemie na chemokuren met Vidaza en daarna met voedingssupplement Metavo. copy 1
- Venetoclax aanvullend op Daunorubicine en Cytarabine als eerstelijnsbehandeling voor patienten met AML - Acute Myeloide Leukemie geeft uitstekende resultaten eerste jaar.
- Myeloablatieve conditionering = platleggen van immuunsysteem door chemo vooraf aan stamceltransplantatie geeft bij Acute Myeloide Leukemie (AML) of MDS - myelodysplastische syndromen veel betere resultaten op overall overleving.
- AML - Acute Myeloide Leukemie: Decitabine - Dacogen - injecties verlengen significant de levensduur en vergroot kans op langdurige remissie van AML
- Haplos = haplo-identieke transplantaties met minder T-cellen bij AML - Actue Myeloide Leukemie vermindert optreden van GvHD met 24 procent en verbetert mediane ziektevrije overleving met 18 procent
- Rydapt (midostaurin) naast chemotherapie geeft langdurige levensverlenging bij acute myeloid leukemia (AML) met FLT3 mutatie.
- AML - acute myeloïde leukemie: Allogene stamceltransplantatie (met donor stamcellen) geeft langere recidiefvrije overleving en betere overall overleving bij patiënten met NPM1-mutatie van acute myeloïde leukemie (AML)
- AML - Acute Myeloide Leukemie: lagere dosis cytarabine geeft zelfde goede resultaten en met minder bijwerkingen bij AML - acute myeloide leukemie blijkt uit grote Nederlandse fase III studie
- AML - Acute Myeloide Leukemie: Onderzoekers aan universiteit van Utah zeggen nieuwe aanpak tegen Acute Myeloide Leukemie te hebben gevonden.
- AML - Acute Myeloide Leukemie: Allogene beenmergtransplantatie geeft significant betere resultaten voor patienten met slechte en matige prognose bij AML - Acute Myolide Leukemie
- AML - Acute Myeloide Leukemie: Gemtuzumab wordt in Amerika vrijwillig uit de markt genomen door Pfizer als aanvullend medicijn naast chemo bij AML - Acute myeloide Leukemie wegens meer sterfte en ernstiger bijwerkingen dan met alleen chemo.
- AML - Acute Myeloide Leukemie, een overzicht van de nieuwste ontwikkelingen



Plaats een reactie ...
Reageer op "Rydapt (midostaurin) naast chemotherapie geeft langdurige levensverlenging bij acute myeloid leukemia (AML) met FLT3 mutatie."