Raadpleeg ook onze lijst van niet-toxische ondersteuning bij prostaatkanker. :
En als donateur kunt u ook korting krijgen bij verschillende bedrijven, waaronder bij Medpro voor o.a. prostasol een veelgebruikt natuurlijk middel bij prostaatkanker als alternatief voor hormoontherapie
9 februari 2021: Zie ook dit artikel: https://kanker-actueel.nl/apalutamide-plus-hormoontherapie-vermindert-het-risico-op-tweede-recidief-of-overlijden-min-33-procent-ongeacht-hormoontherapie-of-chemotherapie-bij-patienten-met-uitgezaaide-hormoonresistente-prostaatkanker.html
21 oktober 2019:
Zie ook dit studierapport: Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer
Dit studierapport analyseerde gegevens uit de tweede vooraf gespecificeerde tussentijdse analyse van een studie waarin apalutamide werd vergeleken met placebo bij patiënten met een hoog risico van niet-uitgezaaide hormoonresistente prostaatkanker. Degenen die met apalutamide werden behandeld, hadden op dit tweede controlepunt een betere overall overleving (HR, 0,75) en progressievrije overleving (HR, 0,55). Dit voordeel werd waargenomen ondanks dat alsnog door een overstap van de behandeling van placebopatiënten naar een behandeling met apalutamide.
Apalutamide lijkt het risico op overlijden van patiënten met niet-uitgezaaide hormoonresistente prostaatkanker te verminderen en kan in de toekomst een haalbare behandelingsoptie zijn voor deze patiëntenpopulatie.
6 december 2018: The Lancet
In The Lancet is deze maand het abstract van de SPARTAN studie met Apalutamide: Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial gepubliceerd. zie hieronder de resultaten. Het abstract staat onderaan dit artikel.
Dit studierapport: Recent trends in the management of advanced prostate cancer geeft een overzicht van de behandelingen bij prostaatkanker op dit moment d.d. 2018. Zie onderaan abstract van de studie en referentielijst
Table 1.
| Trial | Year | Agent | Population | Primary endpoint | Outcome summary |
|---|---|---|---|---|---|
| CHAARTED | 2015 | Docetaxel | Castration-sensitive prostate cancer (CSPC) |
Overall survival (OS) | 13.6-month OS advantage |
| STAMPEDE | 2016 | Docetaxel | CSPC | OS | 15.6-month OS advantage |
| LATITUDE | 2017 | Abiraterone | CSPC | OS | 7% 3-year OS advantage |
| STAMPEDE | 2017 | Abiraterone | CSPC | OS | 17% 3-year OS advantage |
| SPARTAN | 2018 | Apalutamide | M0 castration-resistant prostate cancer (M0 CRPC) |
Metastasis-free survival (MFS) |
24.3-month MFS benefit |
| PROSPER | 2018 | Enzalutamide | M0 CRPC | MFS | 21.9-month MFS benefit |
7 februari 2018: Bron: 2018 Genitourinary Cancers Symposium in San Francisco (Abstract 161).
Apalutamide in tabletvorm, een zogeheten CYP17 remmer zoals bv abiraterone, geeft 24,3 maanden langere ziektevrije tijd (72 procent) in vergelijking met een placebo bij patiënten met prostaatkanker met een hoog risico op ziekteprogressie en uitzaaiingen door stijgende PSA waarden en hormoonresistentie. De onderzoekers van de fase III studie SPARTAN zijn enthousiast over deze resultaten omdat apaluzitamide weinig bijwerkingen geeft en blijkbaar toch weer wat anders werkt dan abiraterone, dat overigens 38 procent betere ziektevrije overleving gaf bij dezelfde groep van patiënten(zie: https://kanker-actueel.nl/abiraterone-acetate-zytiga-geeft-38-procent-minder-kans-op-overlijden-bij-nieuwe-diagnose-van-reeds-uitgezaaide-prostaatkanker-en-verdubbelt-progressievrije-ziekte-van-15-naar-33-maanden.html ) en enzalutamide, dat wel ook superieur was aan casodex / bicalutamide (zie o.a. https://kanker-actueel.nl/enzalutamide-is-ook-superieur-aan-bicalutamide-casodex-bij-prostaatkankerpatienten-ziektevrije-tijd-stijgt-mediaan-van-58-naar-157-maanden-psa-stijging-28-maanden-versus-nog-niet-bereikt-na-20-maanden.html.
Die wel goedgekeurd zijn bij uitgezaaide prostaatkanker maar nog niet bij niet-uitgezaaide prsotaatkanker. Apalutamide heeft hetzelfde werkingsmechanisme als enzalutamide maar heeft wel voordelen ten opzichte van enzalutamide door de lagere doses die nodig zijn, lees ook in Wikipedia
"Tot aan dit onderzoek waren er geen geneesmiddelen waarvan bewezen is dat ze echt een significante verbetering geven aan mannen met niet-uitgezaaide prostaatkanker, ondanks standaard hormonale therapie. Deze resultaten tonen aan dat apalutamide een significant verschil maakte in het verlengen van de tijd vóór de ontwikkeling van uitzaaiingen, "zegt hoofdonderzoeksauteur Eric J. Small, MD, FASCO, Professor of Medicine at the University of California, San Francisco. Ik wil daarbij de kanttekening maken dat er nog geen studieresultaten bekend zijn maar zoals eerder hierboven aangegeven ook enzalutamide en abiraterone geven veel betere resultaten dan casodex. Maar omdat er nog geen resultaten uit recente studies voorhanden zijn heeft de FDA deze ook nog geen goedkeuring gegeven voor deze groep van patiënten.
De FDA heeft voor deze groep van prostaatkankerpatiënten nog nooit een medicijn goedgekeurd, aldus de onderzoekers. Tekst gaat verder onder grafiek van CYP17 remmers en het werkingsmechanisme
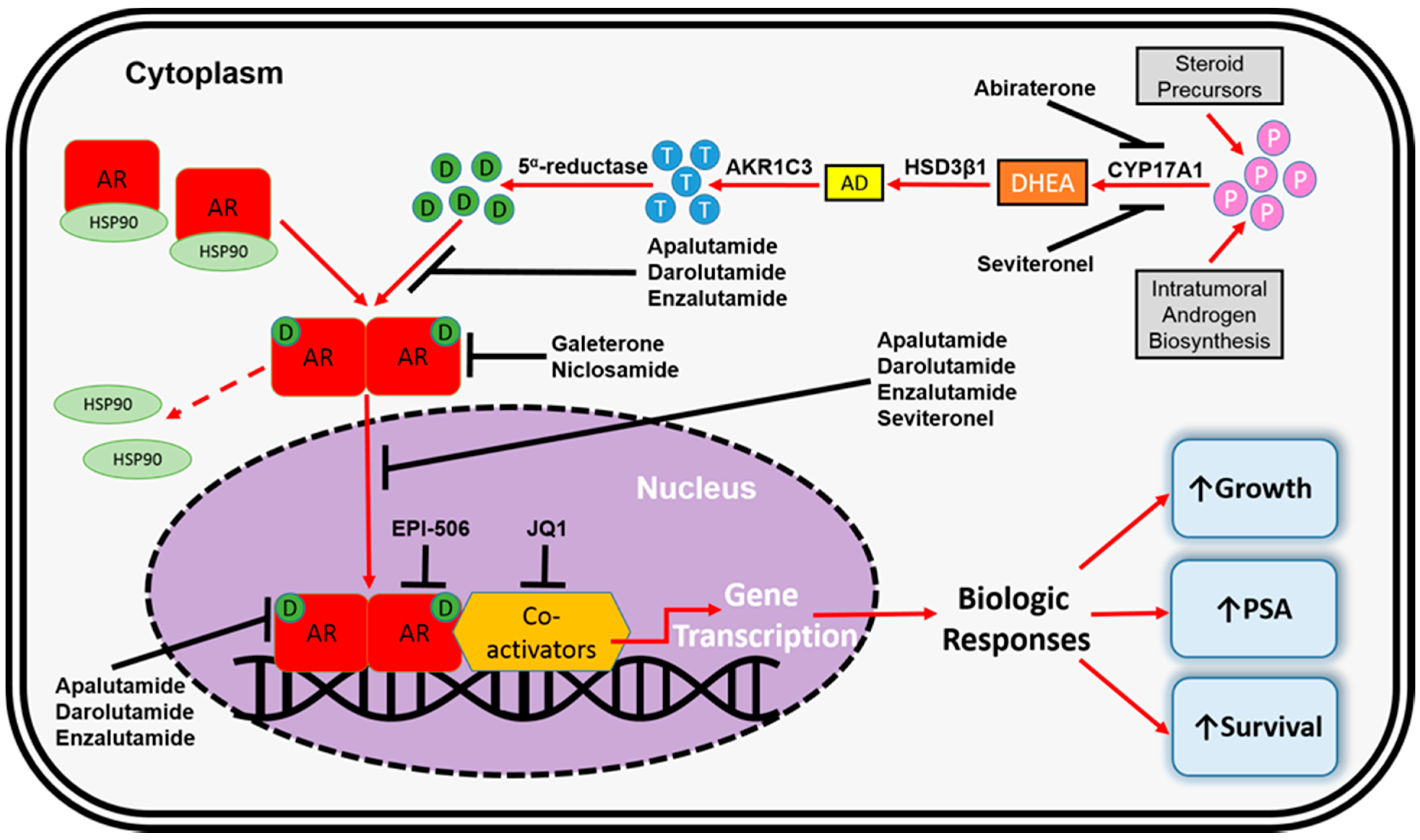
In een eerdere fase II studie gaf apalutamide ook veel betere resultaten in vergelijking met bicalutamide (Casodex) (Comparison of the effect of the antiandrogen apalutamide (ARN-509) versus bicalutamide on the androgen receptor pathway in prostate cancer cell lines.
Er is officieel nog geen abstract gepubliceerd van de SPARTAN studie maar wordt tijdens het 2018 Genitourinary Cancers Symposium gepresenteerd.
Kernpunten uit de studie uit het persbericht met de tussenresultaten zijn veelbelovend:
- Apalutamide verlaagde het risico op uitzaaiingen en overlijden met 72% in vergelijking met placebo en verlengde de mediane uitzaaiingsvrije overleving met 2 jaar, dit is statistisch significant (40,5 maanden in de apalutamidegroep versus 16,2 maanden in de placebogroep).
- Bij deze tussentijdse analyse voor de algehele overleving merkten onderzoekers ook een trend op ter verbetering van de algehele overleving voor mannen die apalutamide versus placebo kregen, hoewel het verschil (nog) niet statistisch significant was.
Hier een beschrijving van hoe de studie was opgezet:
The SPARTAN study was conducted at 332 institutions worldwide and enrolled 1,207 men. Patients with nonmetastatic castration-resistant prostate cancer who had stopped responding to ADT and were at high risk of metastasis based on a PSA doubling time of 10 months or less were randomly assigned to receive apalutamide or placebo taken as daily tablets, added to ongoing ADT. At the time of development of metastases, patients were treated with standard second therapies at their physician’s discretion and had an option to receive on-study abiraterone acetate (Zytiga) and prednisone, a standard of care.
The median PSA doubling time at study entry was approximately 4.5 months in both the apalutamide and placebo groups.
Apalutamide was well tolerated, with 10.7% of men discontinuing treatment due to adverse events compared with 6.3% of men receiving placebo. Quality-of-life scores were maintained in those men receiving apalutamide added to ADT.
Zodra het abstract of volledige studierapprot beschikbaar is zal ik dat erbij plaatsen.
patients who received apalutamide had longer metastasis-free survival and longer time to symptomatic progression than did those who received placebo, while preserving HRQOL
Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial.
Abstract
BACKGROUND:
In the SPARTAN trial, addition of apalutamide to androgen deprivation therapy, as compared with placebo plus androgen deprivation therapy, significantly improved metastasis-free survival in men with non-metastatic castration-resistant prostate cancer who were at high risk for development of metastases. We aimed to investigate the effects of apalutamide versus placebo added to androgen deprivation therapy on health-related quality of life (HRQOL).
METHODS:
SPARTAN is a multicentre, international, randomised, phase 3 trial. Participants were aged 18 years or older, with non-metastatic castration-resistant prostate cancer, a prostate-specific antigen doubling time of 10 months or less, and a prostate-specific antigen concentration of 2 ng/mL or more in serum. Patients were randomly assigned (2:1) to 240 mg oral apalutamide per day plus androgen deprivation therapy, or matched oral placebo plus androgen deprivation therapy, using an interactive voice randomisation system. Permuted block randomisation was used according to the three baseline stratification factors: prostate-specific antigen doubling time (>6 months vs ≤6 months), use of bone-sparing drugs (yes vs no), and presence of local-regional nodal disease (N0 vs N1). Each treatment cycle was 28 days. The primary endpoint was metastasis-free survival. The trial was unblinded in July, 2017. In this prespecified exploratory analysis we assessed HRQOL using the Functional Assessment of Cancer Therapy-Prostate (FACT-P) and EQ-5D-3L questionnaires, which we collected at baseline, day 1 of cycle 1 (before dose), day 1 of treatment cycles 1-6, day 1 of every two cycles from cycles 7 to 13, and day 1 of every four cycles thereafter. This study is registered with ClinicalTrials.gov, number NCT01946204.
FINDINGS:
Between Oct 14, 2013, and Dec 15, 2016, we randomly assigned 1207 patients to receive apalutamide (n=806) or placebo (n=401). The clinical cutoff date, as for the primary analysis, was May 19, 2017. Median follow-up for overall survival was 20·3 months (IQR 14·8-26·6). FACT-P total and subscale scores were associated with a preservation of HRQOL from baseline to cycle 29 in the apalutamide group; there were similar results for EQ-5D-3L. At baseline, the mean for FACT-P total score in both the apalutamide and placebo groups were consistent with the FACT-P general population norm for US adult men. Group mean patient-reported outcome scores over time show that HRQOL was maintained from baseline after initiation of apalutamide treatment and was similar over time among patients receiving apalutamide versus placebo. Least-squares mean change from baseline shows that HRQOL deterioration was more apparent in the placebo group.
INTERPRETATION:
In asymptomatic men with high-risk non-metastatic castration-resistant prostate cancer, HRQOL was maintained after initiation of apalutamide treatment. Considered with findings from SPARTAN, patients who received apalutamide had longer metastasis-free survival and longer time to symptomatic progression than did those who received placebo, while preserving HRQOL.
FUNDING:
Janssen Research & Development.
Copyright © 2018 Elsevier Ltd. All rights reserved.
- PMID:
- 30213449
- DOI:
- 10.1016/S1470-2045(18)30456-X
we review the most recent studies leading to these significant changes in the treatment of advanced prostate cancer
Table 1.
| Trial | Year | Agent | Population | Primary endpoint | Outcome summary |
|---|---|---|---|---|---|
| CHAARTED | 2015 | Docetaxel | Castration-sensitive prostate cancer (CSPC) |
Overall survival (OS) | 13.6-month OS advantage |
| STAMPEDE | 2016 | Docetaxel | CSPC | OS | 15.6-month OS advantage |
| LATITUDE | 2017 | Abiraterone | CSPC | OS | 7% 3-year OS advantage |
| STAMPEDE | 2017 | Abiraterone | CSPC | OS | 17% 3-year OS advantage |
| SPARTAN | 2018 | Apalutamide | M0 castration-resistant prostate cancer (M0 CRPC) |
Metastasis-free survival (MFS) |
24.3-month MFS benefit |
| PROSPER | 2018 | Enzalutamide | M0 CRPC | MFS | 21.9-month MFS benefit |
Recent trends in the management of advanced prostate cancer
Abstract
Advanced prostate cancer includes a wide spectrum of disease ranging from hormone naïve or hormone sensitive to castration resistant, both containing populations of men who have demonstrable metastatic and non-metastatic states. The mainstay of treatment for metastatic hormone-sensitive prostate cancer is androgen deprivation therapy (ADT). However, recent level 1 evidence demonstrates that the addition of chemotherapy or abiraterone acetate to ADT results in significant survival advantage as compared with ADT alone. Furthermore, in non-metastatic castration-resistant prostate cancer (M0 CRPC), two second-generation anti-androgens, apalutamide and enzalutamide, when used in combination with ADT, have demonstrated a significant benefit in metastasis-free survival. Here, we review the most recent studies leading to these significant changes in the treatment of advanced prostate cancer.
Conclusions
There have been significant recent strides in the management of advanced prostate cancer. Major changes in the treatment of hormone-sensitive disease have occurred on the basis of level 1 evidence to support upfront use of docetaxel plus ADT and in addition the use of androgen annihilation with abiraterone acetate plus prednisone in combination with ADT. Also, in the M0 CRPC state, there are now two randomized trials demonstrating improved MFS with the addition of apalutamide or enzalutamide in combination with ADT for patients at high risk for metastases. PARP inhibitors and immunotherapeutic agents such as CTLA-4 inhibitors are also being studied and may become a part of the treatment armamentarium in the near future.
The referees who approved this article are:
-
Axel Heidenreich, Klinik für Urologie, Uro-Onkologie, Roboter-assistierte und Spezielle Urologische Chirurgie, Universitätsklinikum Köln, Köln, GermanyNo competing interests were disclosed.
-
Alicia K Morgans, Division of Hematology/Oncology and Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine, Chicago, IL, USACompeting interests: Consulting honoraria from Janssen, Sanofi, Bayer, Genentech, and Astra Zeneca.
-
Christopher B Anderson, Department of Urology, Herbert Irving Cancer Center, Columbia University Medical Center, New York, New York, USANo competing interests were disclosed.
References
Articles from F1000Research are provided here courtesy of F1000 Research Ltd
Gerelateerde artikelen
- Apalutamide geeft hele goede resultaten op PSA waarden (respons van 85 tot 92 procent) bij hormoonresistente niet uitgezaaide prostaatkanker en hoge therapietrouw.
- Apalutamide en abiraterone acetaat plus prednison vermindert bij patiënten met chemo-naïeve uitgezaaide castratieresistente prostaatkanker met 31 procent het risico op overlijden dan met alleen abiraterone acetate plus prednison copy 1
- Biomoleculaire karakteristieken van prostaattumoren bepalen succes behandeling van apalutamide bij niet uitgezaaide castratieresistente prostaatkanker (nmCRPC)
- apalutamide zorgt voor 2 jaar langere ziektevrije tijd (40.5 vs 16.2 maanden = 72 procent) bij hormoonresistente niet zichtbaar uitgezaaide prostaatkanker met oplopende PSA waarden
- Apalutamide plus hormoontherapie vermindert het risico op tweede recidief of overlijden (min 33 procent) ongeacht hormoontherapie of chemotherapie bij patiënten met uitgezaaide hormoonresistente prostaatkanker



Plaats een reactie ...
Reageer op "apalutamide zorgt voor 2 jaar langere ziektevrije tijd (40.5 vs 16.2 maanden = 72 procent) bij hormoonresistente niet zichtbaar uitgezaaide prostaatkanker met oplopende PSA waarden"