Helpt u ons kanker-actueel online te houden door een donatie?
Raadpleeg ook onze literatuurlijsten
8 januari 2018: lees ook dit artikel:
https://kanker-actueel.nl/het-voordeel-van-immunotherapeutische-combinatie-behandelingen.html
8 januari 2018: klik op de titel voor het originele artikel:
Comprehensive analysis of the clinical immuno-oncology landscape
Wereldwijd zijn een aantal medicijnen reeds geregistreerd voor gebruik binnen immuuntherapie. Nog veel meer medicijnen worden in fase II en III studies onderzocht. Maar uit een onderzoek blijkt dat veel medicijnen zich richten op dezelfde mutaties en DNA afwijkingen waardoor veel dubbel onderzoek plaatsvindt. De onderzoekers van deze studie pleiten voor bundeling van onderzoek naar immuuntherapeutische medicijnen
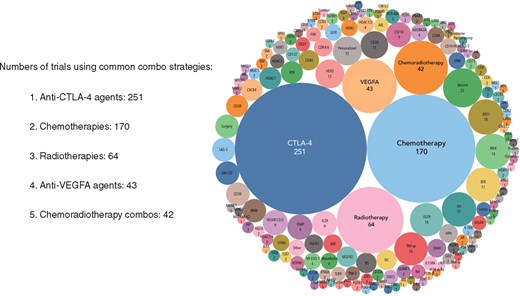
De 940 klinische fase IO-medicijnen (IO = immuno oncology agents) worden uitgevoerd door 462 verschillende bedrijven of academische instellingen (supplementary Table S2, online beschikbaar op Annals of Oncology) en deze medicijnen richten zich op 271 verschillende doelen. Interessant is dat een nader onderzoek van de 940 medicijnen aan het licht bracht dat bijna de helft slechts 40 targets moduleert (Figure 2. We hebben bijvoorbeeld 164 medicijnen gevonden die zich richten op PD-1 of PD-L1 (PD-1 / L1), met 50 medicijnen in onderzoek in de klinische praktijk gericht tegen PD-1 / L1, waarvan 34 monoklonale antilichamen zijn (Figuur 3). Dit bevestigt onze veronderstelling van significante duplicatie, ondanks het feit dat vijf anti-PD-1 / L1 monoklonale antilichamen al zijn goedgekeurd [9]. Hoewel het nog steeds onduidelijk is of het richten op de PD-1-receptor of het ligand PD-L1 zal resulteren in verschillen in werkzaamheid en veiligheid, toont geen van de medicijnen of onderzoeken in onze database een beoordeling van het een-op-een klinische effect bij patiënten. In een ander voorbeeld vertegenwoordigen middelen die zich richten op niet-gespecificeerde tumor-geassocieerde antigenen (niet-gespecificeerd TAA) het grootste aantal klinische IO-medicijnen. Er zijn nu 114 medicijnen in onderzoek ijn klinische studies, waarvan 98 kankervaccins zijn. Van de 114 niet-specifieke TAA-gerichte IO-medicijnen is alleen sipuleucel-T (een autoloog dendritisch celvaccin) goedgekeurd voor prostaatkanker . Dit grote aantal medicijnen roept vragen op over de efficiëntie van middelen en, belangrijker, de patiënten die zich inschrijven voor klinische studies. De concentratie van immuuntherapeutische ontwikkeling en patiëntenbronnen op een paar doelen, sommige met al goedgekeurde geneesmiddelen, kan mogelijk toekomstige innovatie blokkeren. Integendeel, door investeren in deze kostbare hulpbronnen efficiënter te bundelen, kan de benodigde vooruitgang bij het vinden van genezing van deze dodelijke ziekte worden versneld.
Hier nog enkele tabellen uit dit gratis te lezen studierapport: Comprehensive analysis of the clinical immuno-oncology landscape
A large number of IO drugs in clinical development with significant duplication
Overview of the Clinical Accelerator IO database
Our database included 2004 IO agents as of September 2017, 940 of which are in clinical development. On the basis of different mechanisms of actions, we classified these agents into six categories: (i) T-cell-targeted immunomodulators that act on inhibitory or activating molecules expressed by T cells (e.g. agents targeting CTLA-4, PD-1, CD40, and GITR); (ii) other immunomodulators that act on other immune cells or the tumor immune microenvironment to unleash antitumor immunity (e.g. agents modulating IFNAR, CSF1R, IDO1, A2AR, and KIR); (iii) cancer vaccines that induce antigen-specific antitumor immunity (e.g. sipuleucel-T); (iv) cell therapies that engineer immune cells such as T cells to directly attack cancer cells (e.g. anti-CD19 CAR-T); (v) oncolytic viruses that rely on both direct tumor killing and activation of antitumor immunity (e.g. T-VEC); and (vi) CD3-targeted bispecific antibodies that bring T cell to the targeted tumor cells for direct killing (e.g. blinatumomab) (Figure 1).

The overview of 2004 immuno-oncology (IO) agents. Six classes of IO agents are identified on the basis of different mechanisms of actions.>>>>>>>>Reed more
The list of 26 approved immuno-oncology agents, company origins, and targets
| Therapy type | Therapy name | Company | Target |
|---|---|---|---|
| T-cell-targeted immunomodulatory (six in total) | Ipilimumab | Bristol-Myers Squibb Co. | CTLA-4 |
| Nivolumab | Bristol-Myers Squibb Co. | PD-1 | |
| Pembrolizumab | Merck & Co Inc. | PD-1 | |
| Atezolizumab | Roche/Genentech Ltd | PD-L1 | |
| Avelumab | Merck KGaA | PD-L1 | |
| Durvalumab | AstraZeneca/MedImmune LLC | PD-L1 | |
| Other immunomodulatory (eight in total) | Aldesleukin | Novartis AG | IL2R |
| Imiquimod | Valeant Pharmaceuticals Intl Inc. | TLR7 | |
| Interferon alfa | Sumitomo Dainippon Pharma Co Ltd | IFNAR1; IFNAR2 | |
| Interferon alfa-1b | Shenzhen Kexing Biotech Co Ltd | IFNAR1 | |
| Interferon alfa-2a | Cadila Healthcare Ltd | IFNAR1; IFNAR2 | |
| Interferon alfa-2b | Merck & Co Inc. | IFNAR1; IFNAR2 | |
| Interferon beta | Toray Industries Inc. | IFNAR1 | |
| Interferon gamma-1a | Otsuka Pharmaceutical Co Ltd | IFNAR1 | |
| Cancer vaccine (seven in total) | BCG Live | Shire Plc | TLR |
| ImmuCyst | Sanofi | TLR | |
| Immuno BCG | Ataulpho Paiva Foundation | TLR | |
| Mycidac-C | Cadila Pharmaceuticals Ltd | TLR2 | |
| Sipuleucel-T | Dendreon | Unspecified TAA | |
| TICE BCG | Merck & Co Inc. | TLR | |
| Uro-BCG | Medac Inc. | TLR | |
| Cell therapy (two in total) | Tisagenlecleucel | Novartis AG | CD19 |
| Axicabtagene ciloleucel | Gilead | CD19 | |
| Oncolytic virus (two in total) | Oncorine | Shanghai Sunway Biotech Co Ltd | CD40L |
| Talimogene laherparepvec | Amgen Inc. | GMCSFR | |
| CD3-targeted bispecific ab | Blinatumomab | Amgen Inc. | CD19 X CD3 |
Abstract
Advances from immuno-oncology (IO) are changing the standard of care of many types of cancer, and the paradigm of cancer treatments and drug development is being rewritten on a regular basis. Moreover, an unprecedented number of new investigational agents and companies are entering the field of IO. As such, it has become challenging for oncology physicians conducting clinical trials, industry veterans developing IO drugs, and even regulators reviewing novel IO agents to keep track of the rapidly evolving landscape. To help the key stake holders in the field understand the latest IO landscape, we sought to present an unbiased, neutral, scientifically curated, and timely updated analysis of all the current IO agents in clinical development and the clinical trials testing these agents. We based our analyses on information collected from numerous trusted and publicly available sources. We have developed two databases. One database tracks 2004 IO agents (940 in clinical stage and 1064 in preclinical stage) against 303 targets, from 864 companies; the other tracks 3042 active clinical trials of these agents with a target enrollment of 577 076 patients. This report provides key analyses of these data. Furthermore, we will discuss a number of important and actionable trends in the current IO landscape: a large number of companies developing agents against the same IO targets; a rapid increase in the number of anti-PD-1/L1 combination studies, many of which are testing the same combinations and following inefficient patterns; and a significant increase in the number of small, investigator-initiated studies. For each of the findings, we speculate the causes and discuss a few initiatives that aim to address some of these challenges. Finally, by making these landscape analyses available, we aspire to inform the cancer community as they seek to strive for efficiencies and innovation while avoiding duplication.
References
Gerelateerde artikelen
- Nous-209, gepersonaliseerde immuungerichte vaccins tonen veelbelovende resultaten bij het voorkomen van erfelijke kanker bij patiënten met het Lynchsyndroom, waaronder blaaskanker, buikvlieskanker, darmkanker, longkanker
- Poeptransplantatie vergroot effectiviteit van immuuntherapie en vermindert ernstige bijwerkingen bij uitgezaaide gevorderde niercelkanker
- Coronavaccin stimuleert effectiviteit van immuuntherapie en verlengt overleving bij kankerpatienten indien gegeven gelijktijdig of vlak erna naast immuuntherapie
- Oorzaak van spontane kankergenezing lijkt gevonden en het daarop algemene ontwikkelde mRNA vaccin werkte al effectief in dierstudies met melanomen, borstkanker en darmkanker door herprogrammering van afwijkende cellen
- Sucralose, een kunstmatige zoetstof, vermindert effectiviteit van immuuntherapie en geeft slechtere overall overleving bij patienten met verschillende vormen van kanker, waaronder longkanker en melanomen
- Akkermansia muciniphila, een probiotica bacterie, blijkt de effectiviteit van immuuntherapie bij kankerpatienten sterk te verbeteren, blijkt uit meerdere studies
- Oncolytische virussen geven uitstekende resultaten in aanpak van kanker. Clemens Dirven en Casper van Eyck vertellen in Op1 over hun nieuwste vinding waarmee patient met hersentumor is genezen
- Immuuntherapie met gemoduleerde navelstreng natural killer cellen - UBC-nk celtherapie. Wat is dat voor vorm van immuuntherapie?
- Immuuntherapie met anti-PD medicijnen met lagere doses bij kankerpatienten geeft therapeutisch gezien zelfde goede effect als standaard dosering maar veel minder bijwerkingen en kosten zijn veel minder.
- BiTE = Bispecifieke T-cel-engager blijkt hoopvolle nieuwe variant van immuuntherapie met gemoduleerde CAR-T cellen bij de behandeling van kanker. Hier een reviewstudie
- Onderzoekers ontdekken ‘schakelaar’ om apoptose = geprogrammeerde celdood van kankercellen te activeren en lijkt heel belangrijk voor CAR-T celtherapie bij solide tumoren
- Photo Immuno Therapy (PIT) = PDT met infrarood licht blijkt veelbelovende vorm van immuuntherapie voor solide tumoren al of niet in combinatie met andere behandelingen
- Coley vaccin: Immuuntherapie met het Coley vaccin (koorts therapie) stond aan de basis van de huidige immuuntherapie met gemoduleerde virussen en bacterien. Hier een reviewstudie van de Coley therapie
- Darmschimmels - darmbacterien beïnvloeden de immuunreactie na radiotherapie op positieve manier. Doden van darmbacterien door antibiotica heeft negatief effect op immuunreacties na bestraling copy 1
- Een petscan met Novel 18F-Labeled Adnectin kan direct laten zien of een kankerpatient voldoende PD-L1 Expressie heeft voor immuuntherapie met anti-PD medicijnen
- Het voordeel van immunotherapeutische combinatie behandelingen
- Het menselijk immuunsysteem bestaat uit aangeboren immuniteit en verworven immuniteit, maar hebben elkaar nodig voor een goed werkend immuunsysteem. Een uitleg
- Immuuntherapie bij kanker: Grootste doorbraak van 2013 op wetenschappelijk gebied, aldus vakblad Science
- Immuuntherapie met behulp van virussen en antigenen levert hele mooie resultaten op bij verschillende vormen van kanker.
- NLR meting - veranderende verhouding van neutrofielen tot lymfocyten - blijkt een uitstekende en eenvoudige manier om de werkzaamheid van immuuntherapie met anti-PD medicijnen tijdens behandelingsfase te controleren.
- Overzicht van alle wereldwijd geregistreerde medicijnen binnen immuuntherapie en lopende studies met immuuntherapie
- Radiotherapy and MVA-MUC1-IL-2 vaccine act synergistically for inducing specific immunity to MUC-1 tumor antigen
- Rigvir:Immuuntherapie met het gemoduleerde Rigvir virus blijkt bij verschillende vormen van kanker beduidend betere resultaten te geven op overall overleving en complete remissies - genezingen. Een overzichtsstudie van de ontwikkeling van het RIGVIR virus
- Slapende stamcellen: immuuntherapie met oncolytische virussen om recidief te voorkomen via de slapende kankerstamcellen blijkt veelbelovende aanpak. Hier een overzichtsstudie van stand van zaken met deze vorm van immuuntherapie
- Vaccins tegen kanker: Molecuul PRIMA-1 lijkt beschadigde gen P53 tot op DNA niveau te herstellen.
- Virussen: Bewerkt adenovirus - E1B 19kD - rechtstreeks ingebracht in tumorweefsel zorgt voor opmerkelijke resultaten.
- Wat is de juiste patient voor immuuntherapie? Nederlands onderzoek komt tot opmerkelijke conclusies. Internist-oncoloog Hans Westgeest geeft uitleg
- Algemene artikelen over immuuntherapie



Plaats een reactie ...
Reageer op "Overzicht van alle wereldwijd geregistreerde medicijnen binnen immuuntherapie en lopende studies met immuuntherapie"