Helpt u ons aan 500 donateurs?
25 maart 2018: Bron: Irish Journal of Medical Science (1971 -)
Bij ongeveer 10% van de borstkankerpatiënten worden bij de diagnose al uitzaaiingen in andere organen gevonden. Meestal in de longen of botten of de lever. En circa 25% tot 30% van de vrouwen zal na de diagnose ondanks een behandeling toch op termijn uitzaaiingen ontwikkelen in andere organen. Leveruitzaaiingen worden meestal behandeld met systemische chemotherapie en zelden weggehaald met een operatie en andere ablatietechnieken zoals RFA - Radio Frequency Ablation, MWA - Microwave Ablation, LITT - Laser-induced Interstitial Thermotherapy, en meer en meer wordt de laatste jaren de Nanoknife - Ireversible electroporation toegepast, ook bij levertumoren.
Toch blijkt uit een recent gepubliceerde reviewstudie dat in een aantal situaties het zeker zin kan hebben om de leveruitzaaiingen bij borstkanker wel weg te halen. En dan pas systemische chemo te geven of andere vormen van behandelingen, waarbij ik dan zou denken aan immuuntherapie of gerichte behandelingen binnen personalised medicine.
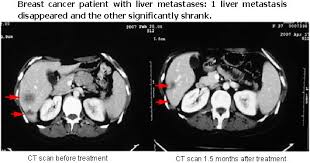
Source image: http://www.genetherapyhospitals.com/BreastCancer/Liver.html
Een recente systemische reviewstudie analyseerde 25 studies die gegevens van totaal 1080 patiënten verwerkte van patiënten met aan borstkanker gerelateerde leveruitzaaiingen.
Van deze patiënten ondergingen er 280 patiënten een leverresectie, de levertumoren werden weggehaald, bv via RFA / MWA of LITT. Deze patiënten hadden een 5-jaars overleving van 24.6% tot 78% en een overall overleving van 29.5 maanden tot 116 maanden. Er ligt dus een groot verschil in die overlevingstijd en belangrijk is wel om de patiënten vooraf goed te selecteren op bepaalde kenmerken schrijven ook de onderzoekers. Van de patiënten met meerdere uitzaaiingen in meerdere organen, oligometastatische ziekte genoemd, waarbij de uitzaaiingen grotendeels waren weggehaald, varieerde de 5-jaars overleving van 21% tot 57% en de algehele overleving varieerde van 32 tot 58 maanden.
Wat mij opvalt is dat de kans om binnen 30 dagen te overlijden gerelateerd aan de operatieve ingreep m.i. hoog ligt, 14% tot 42% voor geïsoleerde en meervoudige metastasen.
Toch concluderen de onderzoekers:
Levertumoren weghalen (Hepatische resectie) kan worden overwogen bij de behandeling van borstkankerpatiënten met geïsoleerde levermetastasen en bij patiënten met oligometastatische ziekte (meerdere uitzaaiingen in meerdere organen).
Uit een andere reviewstudie uit 2011: Resection of liver metastases from breast cancer: Towards a management guideline werden min of meer gelijke resultaten gemeld.
Zie deze grafiek van de studies die daarin werden geanalyseerd en de nieuwe reviewstudie is min of meer een vervolg daarop:
| Author, Journal & Year | study period | Patient Numbers & study design | Complications (m&m) | Survival & outcome |
|---|---|---|---|---|
| Breast only | ||||
| 32Adam R & Aloia T et al; Ann Surg. 2006 | 1984–2004 | 85 breast ca; Single institute | Nil mortality | Median and 5-year overall survivals 46 months and 41% |
| 33Pocard M et al; Eur J Surg Oncol. 2000 |
1988–1997 | 49 breast ca; Single, retrospective data | Nil mortality, 12% morbidity | Survival 86% at 12 months, 79% at 24 months and 49% at 36 months. |
| 34Thelen A et al; J Surg Oncol. 2008 | 1988–2006 | 39 breast cancer | Nil mortality & morbidity 13% | overall 1-, 3-, and 5-year survival 77%, 50%, and 42%, respectively |
| 35Sakamoto Y et al; World J Surg. 2005 | 1985–2003 | 34 breast ca | No mortality | Overall and disease-free 5-year survival 21% and 16%, respectively |
| 36Raab R et al; Anticancer Res 18 (1998) |
11 yrs | 34 breast ca; R0 resection 86%. | Mortality 3% | Overall 5-year survival 18.4% (median 27 months) |
| 37Vlastos G et al; Ann Surg Oncol (2004) |
1991–2002 | 31 breast | No post-operative mortality | The median survival 63 months, Overall 2 and 5-year survival 86% and 61%, respectively |
| 38Yoshimoto M et al; Breast Cancer Res Treat. 2000 | 1985–1998 | 25 breast | Nil | 2 and 5-year cumulative survival 71% and 27%, respectively |
| 23Lubrano J et al; Surg Today. 2008 |
1989–2004 | 16 breast | No death | Overall 1, 3, and 5-year survival 94%, 61%, and 33%, respectively. Median survival 42 months. |
| 39Elias D et al; Am J Surg. 2003 |
1986–2000 | Total 54 patients, 29 breast had surgery only, 25 patients had surgery as well as post-operative Hepatic arterial infusion chemotherapy (HAIC) | morbidity 12.9%; no mortality | 3- and 5-year overall survival 50% and 34% |
| 24Carlini M et al; Hepatogastroenterology 2002 |
Nil available | 17 breast | Mortality nil and morbidity 2 | Actuarial 5-year survival 46%. |
| 25Caralt M et al; Ann Surg Oncol. 2008 |
88–2006 | 12 breast | Nil died, 2 bile leak | Median overall survival 35.9 months. Actuarial 1-, 3-, and 5-year survival 100%, 79%, and 33%, respectively |
| 26Maksan SM et al; Eur. J. Surg. Oncol. 2000 |
1984–1998 | 9 pts breast | No death | 5-year survival 51% |
| 27Seifert JK et al; Hepatogastroenterology 1999 | 1985–1997 | 15 breast | No mortality | Overall median survival following liver resection was 57 months with 1-, 2- and 3-year survival rates of 100%, 71.4% and 53.6% respectively |
| Breast as part of non-colorectal non-neuroendocrine (NCRNE) series | ||||
| 13Adam R et al; Ann Surg. 2006 |
1983–2004 | Total 1452 pts Breast 460 (32%), GI 230 (16%), urologic 206 (14%) & melanoma 148 (10%); 41 French centres; Association of French Surgeons study, R0 resection 83%, preop chemo 42% |
60-day mortality 2.3% and a major complication 21.5% | 5 years Overall and disease-free survival 36% and 21% and at 10 years 23% and 15%, respectively. Tumour recurrence 67% of patients |
| 15Weitz et al; Annals of Surgery 2005 |
1981–2002 | Total 141 patients; Breast 29; melanoma 17; testicular 17; gynaecological 19; (ovarian 12); renal 11; GI 12; Observational study (longitudinal type) |
Post-operative mortality 0%; 46 (33%) post-operative complications |
5 years survival 24% |
| 40Yedibela S et al; Annals of Surgical oncology 2005 | 1978–2001 | Total 152 patients; Stomach 31, pancreas 21, breast 24, SB 17, kidney & GU 27, melanoma 5, sarcoma 8; Single institutional retrospective cohort studies; |
Morbidity 29%, mortality 9% | Overall 2- and 5-year survival 49% and 26%, respectively; Median survival up to 23 months |
| 41Reddy SK et al; J Am Coll Surg 2007 |
1995–2005 | Total 82 patients; Breast 20, ovarian 11, renal 4, sarcoma 19, melanoma 18, gastric 1; retrospective comparative | Mortality 4%, complication 30% | Actuarial 5-year overall and disease-free survival 37% and 16%, respectively. |
| 28O’Rourke TR et al; Annals of Surgical Oncology 2007 | 1986 to 2006 |
Total 102 patients; GU 32 (Renal 16) ovarian 12) melanoma 15, breast 11, sarcoma 3; between 2 hospitals |
Mortality and morbidity 0.8% and 21.1%, respectively | Median survival 42 months and Overall Survival at 3 and 5 years 56.1% and 38.5%, respectively. |
| 17Elias D, Lasser P et al. J Am Coll Surg. 1998 |
1984–1996 | Total 147 patients. 35 breast, 27 neuroendocrine, 20 testicular, 13 sarcomas and 11 gastric, 10 melanomas and 7 gallbladder, 6 gynaecological; single centre | Mortality 2%. | The crude 5-year survival 36% Five-year survival 20% for 35 breast cancers |
| 42Ercolani G et al; Ann Surg Oncol. 2005 | 1990 to 2003 | Total 83 cases gastrointestinal 18, breast 21, genitourinary 15, leiomyosarcoma 10, |
No mortality, 21% morbidity | The 3 and 5-year actuarial survival 49.5% and 34.3%; 3- and 5-year actuarial survival 53.9% and 24.6% from breast cancer |
| 29Earle SA et al; J Am Coll Surg. 2006 | 1990–2005 | Total 76 cases; Pancreas 12, stomach 3; sarcoma 19, breast 10, kidney 10, gynaecological 10, melanoma 4; |
Mortality 2.1%, and post-operative complications 15.8% | Median survival 36 months, and 5-year survival 34.9%. |
| 30Lendoire J et al; HPB, 2007 |
1989 to 2005 | Total 106, renal 21, ovarian 14, sarcoma 23, breast 19, melanoma 6, gastric 3, other GI 4; 5 centres, Cross sectional study |
Perioperative mortality 1.8% | Overall survival 1, 3 and 5 yrs is 67%, 34% and 19% respectively. 5-year survival 53% for breast origin |
| 31Cordera F et al; J Gastrointest Surg 2005 | 1988–1998 | Total 64 patients; GI 12, GU 28, soft tissue 15 (Breast 10), 3 lung; retrospective study at Mayo clinic |
Mortality 1.5% | Actual 1-, 3-, and 5-year survivals 81%, 43%, and 30%, respectively |
| Studies not included: having less than 10 patients of breast ca | ||||
| 19Harrison et al; Surgery 1997 | 1980–1995 | Total 96 patients; sarcoma 27, melanoma 7, breast 7, testicular 9, adrenal 7, renal 5, ovary 7, gastric 5, 8 unknown; Cross Sectional study |
No post-operative complications, but no details given on death | Survival at 1, 3 and 5 yrs 80%, 45% and 37% respectively |
| 20Karavias et al; European Journal of Surgical Oncology 2002 |
1994–2000 | Total 18 patients; Breast 4, kidney 6; gastric 4; intestinal leomyosarcoma 2; Observational (longitudinal type) |
3 cases: pulmonary atelectasis and bile leakage | Median survival 3.2 years |
| 21Benevento A et al; J Surg Oncol. 2000 | 1988–1998 | Total 18 patients; breast 4, gastric 5 | Nil mortality, 8 complications | Overall actuarial survival 54% at 1 year, 42% at 2 years, and 21% at 5 years |
| 22Goering JD et al; Am J Surg. 2002 | 1991–2001 | Total 42 (13 neuroendocrine); 3 renal, 8 ovarian, sarcoma 10, breast 3, melanoma 2 | 1 operative mortality (2%) | Overall survival rates at 1, 3, and 5 years are 82%, 55%, and 39%, respectively (median survival, 45 months). |
| 16Laurent C et al; World J Surg. 2001 |
1980–1997 | Total 39; gastrointestinal 15, genitourinary 12, breast 2, sarcoma 3 |
No mortality | Survival at 1, 3, and 5 years 81, 40, and 35%, respectively; |
| 14Hemming et al; Liver transplantation 2000 |
1978– 1998 |
Total 37 patients; 7 pts GI, 7 sarcoma, 7 renal, 5 melanoma; 2 pancreas; breast 1; Observational study (longitudinal type) |
No surgical deaths. No complications mentioned |
Survival at 1, 3 and 5 yrs is 85%, 55% and 45% respectively; average survival 46 months |
Het volledige studierapport The role of liver resection in patients with metastatic breast cancer: a systematic review examining the survival impact is tegen betaling en door artsen en chirurgen werkzaam in een ziekenhuis gratis in te zien. Het abstract met referentielijst staat hieronder.
Hepatic resection can be considered in the management of breast cancer patients with isolated liver metastases as well as those with oligometastatic disease.
Irish Journal of Medical Science (1971 -)
Tasleem, S., Bolger, J.C., Kelly, M.E. et al. Ir J Med Sci (2018). https://doi.org/10.1007/s11845-018-1746-9
The role of liver resection in patients with metastatic breast cancer: a systematic review examining the survival impact
- 87 Downloads
- 1 Citations
Abstract
Introduction
Approximately 10% of breast cancer patients will present with solid organ metastases, while up to 30% will develop metastatic disease during their treatment course. Liver metastases are usually treated with systemic chemotherapy. Although colorectal liver metastases are routinely resected, this is not yet the standard of care for breast cancer-related liver metastases. This review examines the evidence for resection of breast cancer-related liver metastases.
Methods
A systematic review identified 25 articles for inclusion, 12 papers concerning patients with isolated liver metastases, and 13 papers concerning patients with extrahepatic metastases. Data from 1080 patients were included.
Results
Two hundred eighty patients underwent hepatic resections for breast cancer-associated metastases with no extrahepatic metastases. Reported 5-year survival ranged from 24.6 to 78%. Median overall survival ranged from 29.5 to 116 months. For patients with oligometastatic disease undergoing resection, 5-year survival ranged from 21 to 57%, with median overall survival ranging from 32 to 58 months. Reported 30-day morbidity ranged from 14 to 42% for isolated and multiple metastases.
Conclusion
Hepatic resection can be considered in the management of breast cancer patients with isolated liver metastases as well as those with oligometastatic disease.
References
-
1.Siegel RL, Miller KD, Jemal A (2017) Cancer statistics. CA J Clin 67(1):1–85CrossRefGoogle Scholar
-
2.Cordoso F, Fallowfeild L, Costa A, Castiglione M, Senkus E et al (2012) Locally recurrent or metastatic breast cancer. ESMO clinical practice guidelines, treatment and follow-up. Ann Oncol 23(7):11–19Google Scholar
-
3.Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH (2010) Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol 21(11):2169–2174. https://doi.org/10.1093/annonc/mdq220 PubMedPubMedCentralCrossRefGoogle Scholar
-
4.Fodor J, Major T, József T, Zoltán S, Csaba P (2011) Comparison of mastectomy with breast-conserving surgery in invasive lobular carcinoma: 15-year results. Rep Pract Oncol Radiother 16(6):227–231. https://doi.org/10.1016/j.rpor.2011.06.005 PubMedPubMedCentralCrossRefGoogle Scholar
-
5.Bacalbasa N, Balescu I, Dima S, Popescu I (2015) Long-term survivors after liver resection for breast cancer liver metastases. Anticancer Res 35(12):6913–9617PubMedGoogle Scholar
-
6.Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL (2006) Metastatic patterns in adenocarcinoma. Cancer 106(7):1624–1633. https://doi.org/10.1002/cncr.21778 PubMedCrossRefGoogle Scholar
-
7.Berman AT, Thukral AD, Hwang WT, Solin LJ, Vapiwala N (2013) Incidence and patterns of distant metastases for patients with early-stage breast cancer after breast conservation treatment. Clinical Breast Cancer 13(2):88–94. https://doi.org/10.1016/j.clbc.2012.11.001 PubMedCrossRefGoogle Scholar
-
8.Lee YT (1984) Breast carcinoma: pattern of recurrence and metastasis after mastectomy. Am J Clin Oncol 7:443–449PubMedCrossRefGoogle Scholar
-
9.Singletary SE, Walsh G, Vauthey JN, Curley S, Sawaya R, Weber KL, Meric F, Hortobágyi GN (2003) A role for curative surgery in the treatment of selected patients with metastatic breast cancer. Oncologist 8(3):241–251. https://doi.org/10.1634/theoncologist.8-3-241 PubMedCrossRefGoogle Scholar
-
10.Campos SM, Guastalla JP, Subar M, Abreu P, Winer EP, Cameron DA (2009) A comparative study of exemestane versus anastrozole in patients with postmenopausal breast cancer with visceral metastases. Clin Breast Cancer 9(1):39–44. https://doi.org/10.3816/CBC.2009.n.007 PubMedCrossRefGoogle Scholar
-
11.Crump M, Gluck S, Tu D et al (2008) Randomized trial of high-dose chemo-therapy with autologous peripheral-blood stem-cell support compared with standard-dose chemotherapy in women with metastatic breast cancer: NCIC MA.16. J Clin Oncol 26(1):37–43PubMedCrossRefGoogle Scholar
-
12.Pockaj BA, Wasif N, Dueck A et al (2010) Metastasectomy and surgical resection of the primary tumor in patients with stage IV breast cancer. Ann Surg Oncol 17(9):2419–2426. https://doi.org/10.1245/s10434-010-1016-1 PubMedPubMedCentralCrossRefGoogle Scholar
-
13.Malassagne B, Goere D, Cherqui D, Fagniez PL (2000) Surgical treatment of non-colorectal and non-endocrine liver metastases. Gastroenterology Clin. Biol 24:1177–1185Google Scholar
-
14.Poon RT, Fan ST, Lo CM et al (2004) Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg 240:698–708PubMedPubMedCentralGoogle Scholar
-
15.Jarnagin WR, Gonen M, Fong Y et al (2002) Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 236(4):397–406PubMedPubMedCentralCrossRefGoogle Scholar
-
16.Abdalla EK, Vauthey JN, Ellis LM et al (2004) Recurrence and outcomes following hepatic resection, radiofrequency ablation and combined resection/ablation for colorectal liver metastasis. Ann Surg 239(6):818–825. https://doi.org/10.1097/01.sla.0000128305.90650.71 PubMedPubMedCentralCrossRefGoogle Scholar
-
17.DeJong MC, Pulitano C, Ribero D et al (2009) Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 250(12):440–448Google Scholar
-
18.Schemmer P, Friess H, Hinz U et al (2006) Stapler hepatectomy is a safe dissection technique: analysis of 300 patients. World J Surg 30:419–430PubMedCrossRefGoogle Scholar
-
19.Mavros MN, de Jong M, Dogeas E et al (2013) Impact of complications on long-term survival after resection of colorectal liver metastases. Br J Surg 100(5):711–718PubMedCrossRefGoogle Scholar
-
20.Vlastos G, Smith DL, Singletary SE, Mirza NQ, Tuttle TM, Popat RJ, Curley SA, Ellis LM, Roh MS, Vauthey JN (2004) Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol 11(9):869–874. https://doi.org/10.1245/ASO.2004.01.007 PubMedCrossRefGoogle Scholar
-
21.Salgado R, Denkert C, Campbell C et al (2015) Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO Trial. JAMA Oncol 1:448–454PubMedPubMedCentralCrossRefGoogle Scholar
-
22.Zhang P, Yin Y, Mo H et al (2016) Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial. Oncotarget 7:60647–60656PubMedPubMedCentralGoogle Scholar
-
23.Schoellhammer HF, Hsu F, Vito C, Chu P, Park J, Waisman J, Kim J (2014) Complete pathologic response of HER2-positive breast cancer liver metastasis with dual anti-HER2 antagonism. BMC Cancer 14(1):242. https://doi.org/10.1186/1471-2407-14-242 PubMedPubMedCentralCrossRefGoogle Scholar
-
24.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that cevaluate healthcare interventions: explanation and elaboration. BMJ 339(jul21 1):b2700. https://doi.org/10.1136/bmj.b2700 PubMedPubMedCentralCrossRefGoogle Scholar
-
25.Bacalbasa N, Dima SO, Purtan-Purnichescu R, Herlea V, Popescu I (2014) Role of surgical treatment in breast cancer liver metastases: a single center experience. Anticancer Res 34(10):5563–5568PubMedGoogle Scholar
-
26.Bockhorn M, Frilling A, Busche C, Fingas C, Molmenti E, Broelsch CE (2010) Outcome after resection of breast cancer liver metastases. Int J Hepatol 1(3):39–43Google Scholar
-
27.Duan XF, Dong NN, Zhang T, Li Q (2012) Comparison of surgical outcomes in patients with colorectal liver metastases versus non-colorectal liver metastases: a Chinese experience. Hepatol Res 42(3):296–303. https://doi.org/10.1111/j.1872-034X.2011.00917.x PubMedCrossRefGoogle Scholar
-
28.Ercolani G, Grazi GL, Ravaioli M, Ramacciato G, Cescon M, Varotti G, del Gaudio M, Vetrone G, Pinna AD (2005) The role of liver resections for noncolorectal, nonneuroendocrine metastases: experience with 142 observed cases. Ann Surg Oncol 12(6):459–466. https://doi.org/10.1245/ASO.2005.06.034 PubMedCrossRefGoogle Scholar
-
29.Kollmar O, Moussavian MR, Richter S, Bolli M, Schilling MK (2008) Surgery of liver metastasis in gynecological cancer—indication and results. Onkologie 31(7):375–379. https://doi.org/10.1159/000135516 PubMedCrossRefGoogle Scholar
-
30.Lendoire J, Moro M, Andriani O, Grondona J, Gil O, Raffin G, Silva J, Bracco R, Podestá G, Valenzuela C, Imventarza O, Pekolj J, de Santibañes E (2007) Liver resection for non-colorectal, non-neuroendocrine metastases: analysis of a multicenter study from Argentina. HPB (Oxford) 9(6):435–439. https://doi.org/10.1080/13651820701769701 CrossRefGoogle Scholar
-
31.Lubrano J, Roman H, Tarrab S, Resch B, Marpeau L, Scotté M (2008) Liver resection for breast cancer metastasis: does it improve survival? Surg Today 38(4):293–299. https://doi.org/10.1007/s00595-007-3617-2 PubMedCrossRefGoogle Scholar
-
32.Martinez SR, Young SE, Giuliano AE, Bilchik AJ (2006) The utility of estrogen receptor, progesterone receptor, and Her-2/neu status to predict survival in patients undergoing hepatic resection for breast cancer metastases. Am J Surg 191(2):281–283. https://doi.org/10.1016/j.amjsurg.2005.08.030 PubMedCrossRefGoogle Scholar
-
33.Polistina F, Fabbri A, Ambrosino G (2013) Hepatic colorectal metastases involving infra-hepatic inferior vena cava in high risk patients for extended resection: an alternative method for achieving radical resection in patient with borderline liver remnant. Indian J Surg 75(3):220–225. https://doi.org/10.1007/s12262-012-0681-7 PubMedCrossRefGoogle Scholar
-
34.Vertriest C, Berardi G, Tomassini F, vanden Broucke R, Depypere H, Cocquyt V, Denys H, van Belle S, Troisi RI (2015) Resection of single metachronous liver metastases from breast cancer stage I- II yield excellent overall and disease-free survival. Single center experience and review of the literature. Dig Surg 32(1):52–59. https://doi.org/10.1159/000375132 PubMedCrossRefGoogle Scholar
-
35.Weinrich M et al (2014) Liver resections of isolated liver metastasis in breast cancer: results and possible prognostic factors. HPB Surg 2014:893829PubMedPubMedCentralCrossRefGoogle Scholar
-
36.Zegarac M, Nikolic S, Gavrilovic D, Jevric M, Kolarevic D, Nikolic-Tomasevic Z, Kocic M, Djurisic I, Inic Z, Ilic V, Santrac N (2013) Prognostic factors for longer disease free survival and overall survival after surgical resection of isolated liver metastasis from breast cancer. J BUON 18(4):859–865PubMedGoogle Scholar
-
37.Adam R, Aloia T, Krissat J, Bralet MP, Paule B, Giacchetti S, Delvart V, Azoulay D, Bismuth H, Castaing D (2006) Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg 244(6):897–907; discussion 907-8. https://doi.org/10.1097/01.sla.0000246847.02058.1b PubMedPubMedCentralCrossRefGoogle Scholar
-
38.Caralt M, Bilbao I, Cortés J, Escartín A, Lázaro JL, Dopazo C, Olsina JJ, Balsells J, Charco R (2008) Hepatic resection for liver metastases as part of the “oncosurgical” treatment of metastatic breast cancer. Ann Surg Oncol 15(10):2804–2810. https://doi.org/10.1245/s10434-008-0072-2 PubMedCrossRefGoogle Scholar
-
39.Dittmar Y, Altendorf-Hofmann A, Schüle S, Ardelt M, Dirsch O, Runnebaum IB, Settmacher U (2013) Liver resection in selected patients with metastatic breast cancer: a single- centre analysis and review of literature. J Cancer Res Clin Oncol 139(8):1317–1325. https://doi.org/10.1007/s00432-013-1440-2 PubMedCrossRefGoogle Scholar
-
40.Groeschl RT, Nachmany I, Steel JL, Reddy SK, Glazer ES, de Jong MC, Pawlik TM, Geller DA, Tsung A, Marsh JW, Clary BM, Curley SA, Gamblin TC (2012) Hepatectomy for noncolorectal non-neuroendocrine metastatic cancer: a multi-institutional analysis. J Am Coll Surg 214(5):769–777. https://doi.org/10.1016/j.jamcollsurg.2011.12.048 PubMedCrossRefGoogle Scholar
-
41.Hoffmann K, Franz C, Hinz U, Schirmacher P, Herfarth C, Eichbaum M, Büchler MW, Schemmer P (2010) Liver resection for multimodal treatment of breast cancer metastases: identification of prognostic factors. Ann Surg Oncol 17(6):1546–1554. https://doi.org/10.1245/s10434-010-0931-5 PubMedCrossRefGoogle Scholar
-
42.Kostov DV, Kobakov GL, Yankov DV (2013) Prognostic factors related to surgical outcome of liver metastases of breast cancer. J Breast Cancer 16(2):184–192. https://doi.org/10.4048/jbc.2013.16.2.184 PubMedPubMedCentralCrossRefGoogle Scholar
-
43.Margonis GA, Buettner S, Sasaki K, Kim Y, Ratti F, Russolillo N, Ferrero A, Berger N, Gamblin TC, Poultsides G, Tran T, Postlewait LM, Maithel S, Michaels AD, Bauer TW, Marques H, Barroso E, Aldrighetti L, Pawlik TM (2016) The role of liver-directed surgery in patients with hepatic metastasis from primary breast cancer: a multi-institutional analysis. HPB (Oxford) 18(8):700–705. https://doi.org/10.1016/j.hpb.2016.05.014 CrossRefGoogle Scholar
-
44.Mariani P, Servois V, de Rycke Y, Bennett SP, Feron JG, Almubarak MM, Reyal F, Baranger B, Pierga JY, Salmon RJ (2013) Liver metastases from breast cancer: surgical resection or not? A case- matched control study in highly selected patients. Eur J Surg Oncol 39(12):1377–1383. https://doi.org/10.1016/j.ejso.2013.09.021 PubMedCrossRefGoogle Scholar
-
45.Ruiz A et al (2015) Repeat hepatectomy for breast cancer liver metastases. Ann Surg Oncol 22(Suppl 3):S1057–S1066PubMedCrossRefGoogle Scholar
-
46.Sabol M, Donat R, Chvalny P, Dyttert D, Palaj J, Durdik S (2014) Surgical management of breast cancer liver metastases. Neoplasma 61(5):601–606PubMedCrossRefGoogle Scholar
-
47.Sakamoto Y, Yamamoto J, Yoshimoto M, Kasumi F, Kosuge T, Kokudo N, Makuuchi M (2005) Hepatic resection for metastatic breast cancer: prognostic analysis of 34 patients. World J Surg 29(4):524–527. https://doi.org/10.1007/s00268-004-7688-6 PubMedCrossRefGoogle Scholar
-
48.van Walsum GA, de Ridder JA, Verhoef C, Bosscha K, van Gulik TM, Hesselink EJ, Ruers TJ, van den Tol MP, Nagtegaal ID, Brouwers M, van Hillegersberg R, Porte RJ, Rijken AM, Strobbe LJ, de Wilt JH, Dutch Liver Surgeons Group (2012) Resection of liver metastases in patients with breast cancer: survival and prognostic factors. Eur J Surg Oncol 38(10):910–917. https://doi.org/10.1016/j.ejso.2012.04.015 PubMedCrossRefGoogle Scholar
-
49.Thelen A, Benckert C, Jonas S, Lopez-Hänninen E, Sehouli J, Neumann U, Rudolph B, Neuhaus P (2008) Liver resection for metastases from breast cancer. J Surg Oncol 97(1):25–29. https://doi.org/10.1002/jso.20911 PubMedCrossRefGoogle Scholar
-
50.Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, Courdi A, Hannoun-Levi JM, Ettore F, Birtwisle-Peyrottes I, Balu-Maestro C, Marcy PY, Raoust I, Lallement M, Chamorey E (2008) Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol 19(12):2012–2019. https://doi.org/10.1093/annonc/mdn424 PubMedPubMedCentralCrossRefGoogle Scholar
-
51.Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S et al (2010) International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst 102(7):456e63CrossRefGoogle Scholar
-
52.Adam R, Chiche L, Aloia T, Elias D, Salmon R, Rivoire M, Jaeck D, Saric J, le Treut YP, Belghiti J, Mantion G, Mentha G, Association Française de Chirurgie (2006) Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 244(4):524–535. https://doi.org/10.1097/01.sla.0000239036.46827.5f PubMedPubMedCentralGoogle Scholar
-
53.Diaz R et al (2004) Hepatic resection in breast cancer metastases: should it be considered standard treatment? Breast 13(3):254–258. https://doi.org/10.1016/j.breast.2003.11.001 PubMedCrossRefGoogle Scholar
-
54.Pogoda K, Niwińska A, Murawska M, Pieńkowski T (2013) Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol 30(1):388. https://doi.org/10.1007/s12032-012-0388-4 PubMedPubMedCentralCrossRefGoogle Scholar
-
55.Rastogi S, Gulia S, Bajpai J, Ghosh J, Gupta S (2014) Oligometastatic breast cancer: a mini review. Indian J Med Paediatr Oncol 35(3):203e6Google Scholar
-
56.Hanrahan EO, Broglio KR, Buzdar AU et al (2005) Combined-modality treatment for isolated recurrences of breast carcinoma: update on 30 years of experience at the University of Texas M.D. Anderson Cancer Center and assessment of prognostic factors. Cancer 1046:1158–1171CrossRefGoogle Scholar
-
57.Yoo TG, Cranshaw I, Broom R, Pandanaboyana S, Barlett A et al (2017) Systematic review of early and long-term outcome of liver resection for metastatic breast cancer: is there a survival benefit? Breast 32:162–172PubMedCrossRefGoogle Scholar
-
58.Chua TC, Saxena A, Liauw W, Chu F, Morris DL (2011) Hepatic resection for metastatic breast cancer: a systematic review. Eur J Cancer 47(15):2282–2290. https://doi.org/10.1016/j.ejca.2011.06.024 PubMedCrossRefGoogle Scholar
-
59.Howlader M, Heaton N, Rela M (2011) Resection of liver metastases from breast cancer: towards a management guideline. Int J Surg 9(4):285–291. https://doi.org/10.1016/j.ijsu.2011.01.009 PubMedCrossRefGoogle Scholar
-
60.Wyld L, Gutteridge E, Pinder SE, James JJ, Chan SY, Cheung KL, Robertson JFR, Evans AJ (2003) Prognostic factors for patients with hepatic metastases from breast cancer. Br J Cancer 89(2):284–290. https://doi.org/10.1038/sj.bjc.6601038 PubMedPubMedCentralCrossRefGoogle Scholar
-
61.Pocard M, Pouillart P, Asselain B, Falcou MC, Salmon RJ (2001) Hepatic resection for breast cancer metastases: results and prognosis (65 cases). Ann Chir 126(5):413–420. https://doi.org/10.1016/S0003-3944(01)00526-0 PubMedCrossRefGoogle Scholar
-
62.Duan XF, Dong NN, Zhang T, Li Q (2013) The prognostic analysis of clinical breast cancer subtypes among patients with liver metastases from breast cancer. Int J Clin Oncol 18(1):26–32. https://doi.org/10.1007/s10147-011-0336-x PubMedCrossRefGoogle Scholar
-
63.Butters BJ, Ghersi D, Wilcken N, Kirk SJ, Mallon PT. Addition of drug/s to a chemotherapy regimen for metastatic breast cancer. Cochrane Database of Systematic Reviews 2010;11. Art No CD003368Google Scholar
-
64.Sadot E, Lee SY, Sofocleous CT, Solomon SB, Gönen M, Peter Kingham T, Allen PJ, DeMatteo RP, Jarnagin WR, Hudis CA, D’Angelica MI (2016) Hepatic resection or ablation for isolated breast cancer liver metastasis: a case-control study with comparison to medically treated patients. Ann Surg 264(1):147–154. https://doi.org/10.1097/SLA.0000000000001371 PubMedPubMedCentralCrossRefGoogle Scholar
-
65.Yersal O, Barutca S (2014) Biological subtypes of breast cancer: prognostic and therapeutic implications. World J Clin Oncol 5(3):412–424. https://doi.org/10.5306/wjco.v5.i3.412 PubMedPubMedCentralCrossRefGoogle Scholar
-
66.Atalay G, Biganzoli L, Renard F, Paridaens R, Cufer T, Coleman R, Calvert AH, Gamucci T, Minisini A, Therasse P, Piccart MJ, EORTC Breast Cancer and Early Clinical Studies Groups (2003) Clinical outcome of breast cancer patients with liver metastases alone in the anthracycline-taxane era: a retrospective analysis of two prospective, randomised metastatic breast cancer trials. Eur J Ca 39(17):2439–2449. https://doi.org/10.1016/S0959-8049(03)00601-4 CrossRefGoogle Scholar
-
67.Yoshimoto M, Tada T, Saito M, Takahashi K, Makita M, Uchida Y, Kasumi F (2000) Surgical treatment of hepatic metastases from breast cancer. Breast Cancer Res Treat 59(2):177–184. https://doi.org/10.1023/A:1006398401352 PubMedCrossRefGoogle Scholar
Copyright information
Gerelateerde artikelen
- Borstreconstructie mag voortaan door plastische chirurgen worden bepaald of het nodig is of niet. Ziektekostenverzekeraars veranderen machtiging tot borstreconstructie.
- SIRA, een RFA elektrochirurgisch apparaat dat intraoperatief RFA geeft bij borstbesparende operatie is door FDA goedgekeurd en eerste borstkankerpatient is er succesvol mee behandeld
- Radio Frequency Ablation (RFA) en Cryosurgery (bevriezingstechniek) blijken ook bij operabele niet uitgezaaide borstkanker uitstekende operatietechnieken
- Borstsparende operatie van mucineuze borstkanker geeft een lager risico op overlijden door welke oorzaak dan ook in vergelijking met volledige borstverwijdering (mastectomy
- Cryosurgery plus 1x ipilimumab vooraf aan operatie van operabele borstkanker stimuleert immuunreactie en geeft betere therapeutische resultaten copy 1
- Eerst operatie van borst voor HER2 positieve borstkanker stadium IV gevolgd door systemische behandelingen geeft 44 procent meer overall overlevingen dan systemische behandelingen zonder operatie
- Weghalen van levertumoren bij geselecteerde groep patienten met uitgezaaide borstkanker geeft langere ziektevrije tijd en betere overall overleving op 5 jaar
- Alleen schildwachtklier verwijderen bij borstkanker geeft na 10 jaar zelfde resultaten op overall overleving en kans op recidief dan volledige okselklierdissectie
- Beste borstkankeroperatie techniek - echogeleide operatie - wordt nog te weinig ingezet in Nederland. 17 procent loopt onnodig risico op recidief of hersteloperatie copy 1
- Borstsparende operatie plus bestraling geeft 20 procent betere 10-jaars ziektevrije overleving dan volledige borstamputatie zonder bestraling voor borstkanker
- CPM - contralaterale profylactische mastectomie - verwijdering van gezonde borst naast operatieve verwijdering van borst met kanker geeft nauwelijks verschil in overlevingskansen
- MarginProbe bewijst grote waarde voor patiënten met operabele borstkanker. Noodzaak van hersteloperatie verminderde door MarginProbe met 50 procent
- Operatie: Een ultra sound - echografisch - geleide operatie lijkt beter in staat om volledig borstkanker tumoren te verwijderen en spaart meer gezond weefsel dan een standaard operatie geleid door palpatie (op gevoel van de chirurg).
- Operatie: Harvard professoren hadden al in 2003 grote twijfels bij bioptie en operatie van borstkanker.
- Cryosurgery - bevriezingstechniek - wordt soms wel toegepast bij borstkanker om een recidief te voorkomen
- Operatie: De kans op een recidief van borstkanker na operatie en schone schildwachtklieren is veel groter dan verwacht blijkt uit grote Zweedse studie.
- Operatie: Een operatieve verwijdering van de primaire tumor bij reeds uitgezaaide borstkanker geeft toch significant betere overlevingskansen, vooral bij vrouwen met alleen uitzaaiingen in de botten.
- Operatie: Routinematige operatie van de okselklieren bij beginnende borstkanker is onnodig en beinvloed kwaliteit van leven onnodig als er verder geen aantoonbare uitzaaiingen zijn en schildwachtklier schoon is. Artikel geplaatst 27 juni 2010
- Operatie: aantal artikelen over operatie technieken en gevolgen van operatieve ingrepen bij borstkanker bij elkaar gezet



Plaats een reactie ...
Reageer op "Weghalen van levertumoren bij geselecteerde groep patienten met uitgezaaide borstkanker geeft langere ziektevrije tijd en betere overall overleving op 5 jaar"