Raadpleeg ook literatuurlijst niet-toxische middelen en behandelingen specifiek bij darmkanker van arts-bioloog drs. Engelbert Valstar.
19 juni 2019: lees ook dit artikel:
25 januari 2019: Zie ook dit artikel:
28 september 2016: Bron: diverse studies en bronnen waaronder eigen bezoek aan Gent
Afgelopen week was ik in Gent met een patiënt met darmkanker met uitzaaiingen in zijn buikvlies om te kijken of een PIPAC behandeling nog iets voor hem zou zijn. PIPAC - Pressurized Intraperitoneal Aerosol Chemotherapy - is het toedienen van vernevelde chemotherapie in de buikholte via een kijkoperatie. PIPAC wordt vooral gebruikt voor buikvliestumoren, buikvlieskanker, blinde darmkanker, uitzaaiingen vanuit eierstokkanker en darmkanker en soms zelfs vanuit maagkanker, alvleesklierkanker en borstkanker. Het gaat dus vooral om de tumoren te bestrijden die zich in het buikvlies bevinden of aan de buitenkant van in de buikholte gelegen organen.
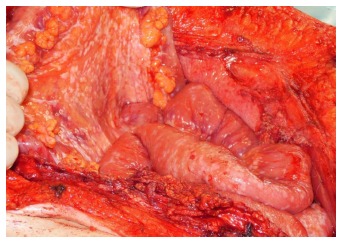
PIPAC een min of meer vereenvoudigde vorm van HYPEC maar dan zonder verwarming - hyperthermie van de chemo is uitgevonden door een arts in Bochum en wordt in Europa alleen gegeven in Bochum en Gent. In principe duurt de PIPAC ca. 4 uur en kan de patiënt als de PIPAC 's morgens wordt gegeven de patient 's avonds weer naar huis. De bijwekringen zijn goed hanteerbaar en de PIPAC wordt door patiënten over het algemeen goed verdragen, aldus dr. Willaerts en blijkt ook uit studies.
Er is al wel onderzoek gedaan maar nog geen gerandomiseerde placebo gecontroleerde studies omdat dat bijna niet mogelijk is en ook ethisch niet verantwoord zou zijn zo vertelde mij dr. Wilaerts, de arts die ik sprak in Gent.
De PIPAC wordt wel gedeeltelijk vergoed door de verzekeraars (ook voor patiënten in Nederland maar wel vantevoren aanvragen) maar de vernevelaar moet wel zelf worden betaald. In Gent kost die ca. € 1.800,--. Een PIPAC is herhaalbaar en ook te combineren met al of niet systemische chemo en wordt als er herhaald kan of moet worden gemiddeld 1x per 6 weken gegeven.
Wat mij wel verbaasde is dat dr. Willaerts nog nooit van hyperthermie had gehoord. Zelfs wist hij niet dat in de Daniel den Hoed, het aAMC en het Verbeeten Instituut moderne hyperthermie machines staan die o.a. veel gebruikt worden voor baarmoederhalskanker, mond- en keelkanker en borstkanker. De nieuwe hyperthermie machines daar die het lichaam omhullen zouden m.i. uitermate geschikt zijn om vooraf en daarna toe te passen samen met de PIPAC. Ik heb in ieder geval de patiënt waarmee ik in Gent was aangeraden en hij ging dat ook doen want had er wel al enige ervaring mee, om de dag voor de PIPAC behandeling electro hyperthermie te doen en wellicht ook enkele dagen erna.
Interessant is ook dat een recente studie nog heeft uitgewezen dat een PIPAC invloed heeft op DNA mutaties die zijn gerelateerd aan immuunreacties. Ik moet zeggen dat de PIPAC een interessante behandeling is m.i.
Hier een aantal studies met de PIPAC, abstracten daarvan staan onderaan dit artikel:
Therapeutic options for peritoneal metastasis arising from colorectal cancer.
En bij eierstokkanker
Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: A phase 2 study.
en bij buikvliestumoren
Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis.
en ook deze bij uitzaaiingen in het buikvlies vanuit maagkanker is interessant:
Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low-Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis
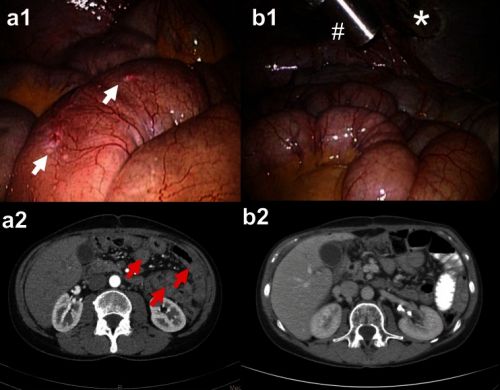
Foto hierboven: Scanbeelden voor en na een PIPAC
Van voorgaande studies zijn ook referentielijsten bekend die onderaan dit artikel staat bij het abstract van de studie.
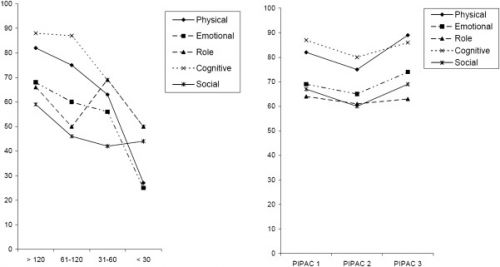
Quality of life of patients with end-stage peritoneal metastasis treated with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC).
Figure 2
EORTC-QLQ30 functional scores. Left panel: in 91 peritoneal carcinomatosis patients classified according to their survival at time of assessment; right panel: in a subgroup of 48 of those patients having received at least 2 PIPAC at 6 weeks intervals. X-axis: days until death. Y-axis: score in %.
PIPAC is well tolerated and active in women with recurent ovarian cancer and warrants further investigation in these patients.
Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: A phase 2 study.
Abstract
OBJECTIVE:
Recurrent ovarian, fallopian or peritoneal cancer with peritoneal carcinomatosis (ROCPC) is resistant to systemic chemotherapy. We assessed the safety and activity of laparoscopic pressurized intraperitoneal aerosol chemotherapy (PIPAC) in women with this cancer.
METHODS:
In this open-label, single-arm phase 2 study, patients underwent 3 courses q 28-42 days of PIPAC with doxorubicin 1·5 mg/m(2) followed by cisplatin 7·5 mg/m(2). A pressure of 12 mm Hg and a temperature of 37 °C were applied for 30 min/course. The primary endpoint was the proportion of patients who had an objective tumor response (OTR) according to RECIST version 1.1 criteria. Analysis was by intention to treat. Secondary endpoints were tumor regression on histology, PC Index improvement on repeated video-laparoscopy, and quality of life measured with the EORTC QLQ-30 questionnaire.
RESULTS:
Sixty-four patients were enrolled. Laparoscopic non-access rate was 11/64 (17%). 53 patients were eligible for analyses. 33/53 (62%) patients had an OTR - three had a partial response and 30 patients had stable disease. Tumor regression on histology and PC Index improvement were observed in 26/34 (76%) and in 26/34 (76%) patients who underwent all 3 PIPACs. There were no treatment-related deaths. No grade 4 toxicity was observed. Grade 3 toxicities were trocar hernia (n=2), bowel obstruction (n=2), abdominal pain (n=2), hematoma (n=1), intraoperative bleeding (n=1), and cystitis with urosepsis (n=1). EORTC QLQ-30 global physical health scores, nausea/vomiting, appetite loss, diarrhea, and constipation improved during therapy.
CONCLUSION:
PIPAC is well tolerated and active in women with ROCPC and warrants further investigation in these patients.
Copyright © 2015 Elsevier Inc. All rights reserved.
KEYWORDS:
Chemotherapy; High pressure; Intraabdominal; Platinum-resistant; Recurrent ovarian cancer
- PMID:
- 25701703
- DOI:
- 10.1016/j.ygyno.2015.02.009
- [PubMed - indexed for MEDLINE]
PIPAC with low-dose cisplatin and doxorubicin was safe and induced objective tumor regression in selected patients with PM from recurrent, platinum-resistant GC. First survival data are encouraging and justify further clinical studies in this indication.
Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low-Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis
Abstract
Background
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a novel technique of intraperitoneal chemotherapy. First results obtained with PIPAC in patients with advanced peritoneal metastasis (PM) from gastric cancer (GC) are presented.
Methods
Retrospective analysis: Sixty PIPAC were applied in 24 consecutive patients with PM from GC. 67 % patients had previous surgery, and 79 % previous platinum-based systemic chemotherapy. Mean Peritoneal Carcinomatosis Index (PCI) of 16 ± 10 and 18/24 patients had signet-ring GC. Cisplatin 7.5 mg/m2 and doxorubicin 1.5 mg/m2 were given for 30 min at 37 °C and 12 mmHg at 6 week intervals. Outcome criteria were survival, adverse events, and histological tumor response.
Results
Median follow-up was 248 days (range 105–748), and median survival time was 15.4 months. Seventeen patients had repeated PIPAC, and objective tumor response was observed in 12 (12/24 = 50 %): no vital tumor cells = 6, major pathological response = 6, minor response = 3. Postoperative adverse events > CTCAE 2 were observed in 9 patients (9/24, 37.5 %). In 3/17 patients, a later PIPAC could not be performed due to non-access. Two patients (ECOG 3 and 4) died in the hospital due to disease progression.
Conclusion
PIPAC with low-dose cisplatin and doxorubicin was safe and induced objective tumor regression in selected patients with PM from recurrent, platinum-resistant GC. First survival data are encouraging and justify further clinical studies in this indication.
Electronic supplementary material
The online version of this article (doi:10.1007/s11605-015-2995-9) contains supplementary material, which is available to authorized users.
References
In summary, molecular changes of peritoneal cancer during pressurized intraperitoneal aerosol chemotherapy can be documented and may be used to refine individual treatment and prognostic estimations.
Dynamic changes of tumor gene expression during repeated pressurized intraperitoneal aerosol chemotherapy (PIPAC) in women with peritoneal cancer.
Abstract
BACKGROUND:
Intraperitoneal chemotherapy is used to treat peritoneal cancer. The pattern of gene expression changes of peritoneal cancer during intraperitoneal chemotherapy has not been studied before. Pressurized intraperitoneal aerosol chemotherapy is a new form of intraperitoneal chemotherapy using repeated applications and allowing repeated tumor sampling during chemotherapy. Here, we present the analysis of gene expression changes during pressurized intraperitoneal aerosol chemotherapy with doxorubicin and cisplatin using a 22-gene panel.
METHODS:
Total RNA was extracted from 152 PC samples obtained from 63 patients in up to six cycles of intraperitoneal chemotherapy. Quantitative real-time PCR was used to determine the gene expression levels. For select genes, immunohistochemistry was used to verify gene expression changes observed on the transcript level on the protein level. Observed (changes in) expression levels were correlated with clinical outcomes.
RESULTS:
Gene expression profiles differed significantly between peritoneal cancer and non- peritoneal cancer samples and between ascites-producing and non ascites-producing peritoneal cancers. Changes of gene expression patterns during repeated intraperitoneal chemotherapy cycles were prognostic of overall survival, suggesting a molecular tumor response of peritoneal cancer. Specifically, downregulation of the whole gene panel during intraperitoneal chemotherapy was associated with better treatment response and survival.
CONCLUSIONS:
In summary, molecular changes of peritoneal cancer during pressurized intraperitoneal aerosol chemotherapy can be documented and may be used to refine individual treatment and prognostic estimations.
References
Gerelateerde artikelen
- Algemene artikelen die met kanker te maken hebben maar niet specifiek bij een vorm van kanker
- Aflibercept (Zaltrap) toegevoegd aan een chemo combinatie van fluorouracil - 5-FU, leucovorine en irinotecan (FOLFIRI) verbetert de overleving en progressievrije tijd met 1,5 maand
- Aprepitant - Emend is een geneesmiddel dat de bijwerkingen van chemokuren vermindert en inmiddels veelgebruikt middel om misselijkheid bij chemo tegen te gaan
- Avastin - Bevacizumab, een angiogenese remmer die bij een aantal vormen van kanker gebruikt wordt inmiddels: overzicht van een aantal studies en belangrijke artikelen
- BBBD-behandeling (Blood Brain Barrier Disruption) bij lymfomen (tumoren) van centrale zenuwstelsel succesvol en hoog significant aldus fase II studie.
- Bloedcellen: Onze longen ondersteunen ons beenmerg in het maken van rode bloedcellen, bloedplaatjes en immuuncellen.
- Beenmergtransplantaties en stamceltransplantaties: Studiepublicaties van niet-toxische middelen en behandelingen uit literatuurlijst van arts-bioloog drs. Engelbert Valstar naast beenmergtransplantaties en stamceltransplantaties
- Bloedarmoede (anemia) en ESA's : Medicijnen die de aanmaak van rode bloedlichaampjes stimuleren en vaak ingezet worden tegen bloedarmoede en moeheid geven een groter risico (17%) om te sterven tijdens de chemo en geeft kortere mediane overlevingstijd.
- Boekenlijst met daarop titels van boeken die op een of andere manier in relatie staan tot kanker en omgaan daarmee
- Celebrex - een zogeheten COX-2 remmer geeft 2,5 keer zo groot risico op ernstige hartklachten aldus gerandomiseerde dubbelblinde langjarige studie. Aandeel van producent Pfizer zakte 17% in waarde op 1 dag.
- Chemo en voedingsondersteuning: een overzicht van een aantal studies en opinies bij elkaar in 1 overzicht. Inclusief studielijst chemo en voedingstoffen
- Clolar - clofarabine plus busulfan geeft uitstekende resultaten bij ALL - Acute lymfatische Leukemie en stamceltransplantatie
- COBALT - Combination Bacteriolytic Therapy - chemo en bacteriën succesvol in dierproeven, maar wordt ook bekritiseerd door hoge toxiciteit
- Cryosurgery bij goedaardige en kleine kwaadaardige bottumoren - chondrosarcomen - wordt vaak succesvol en genezend toegepast.
- Da Vinci robot werkt effectief en nauwkeurig bij o.a. prostaatkanker
- Dendritische celtherapie informatie en studies enz. bij elkaar gezet in 1 artikelenreeks
- Denosumab geeft prostaatkankerpatienten met risico op recidief significant langere tijd tot zich botuitzaaiingen voordoen, maar geen verbetering van overall overleving
- Diagnose: Kanker zonder primaire oorsprong komt minder voor door betere diagnostiek, maar kans op overlijden blijft gelijk.
- Dolasetron, injectie om misselijkheid bij chemo tegen te gaan geeft vergroot risico op hartfalen, waarschuwt de FDA. Dolasetron mag niet meer gebruikt worden in hoge dosis.
- EGRF remmers zoals Tarceva - Erlotinib en Iressa - Gefitinib: een overzicht
- Epigenetische behandeling met Azacitidine en Entinostat blijkt succesvolle aanpak van niet-klein-cellige longkanker
- Erbitux - Cetuximab, een overzicht van belangrijke studies en recente ontwikkelingen..
- Everolimus vermindert sterk epileptische aanvallen bij patiënten met het tubereuze sclerose complex, goedaardige tumoren in de hersenen vanuit erfelijke oorsprong en verbetert kwaliteit van leven aanzienlijk.
- Gamma-Knife - Stereotactische bestraling - informatie bij elkaar gezet
- Gentherapie met p53 virus (gendicine) en hyperthermie zorgt voor spectaculaire verbeteringen bij patienten met vergevorderde kanker blijkt uit kleinschalige fase 2 studie.
- Gleevec - Imatinib: een overzicht van belangrijke studies en recente ontwikkelingen met dit medicijn
- Heparine, laag moleculair gewicht, blijkt overlevingstijd van kankerpatiënten met solide tumoren en zonder uitzaaiïngen significant te beinvloeden, aldus Nederlandse studie
- Hersenmetastases: Hardnekkige misvattingen over behandelen van uitzaaiingen in de hersenen maken dat veel patiënten verkeerd worden behandeld en heeft grote gevolgen voor therapeutisch effect op ziektevrije tijd en overall overleving
- Herceptin - trastuzumab: een overzicht
- HPV test voor vrouwen goedgekeurd door FDA
- HYPEC - Hypertherme Intra Peritoneale Chemotherapie informatie
- Hyperthermie in combinatie met doelgerichte en immuuntherapeutische behandelingen is veel belovende combinatiebehandeling voor kankerpatienten, aldus artsen van ELMEDIX van de universiteit van Antwerpen
- Hyperthermie: Electromagnetische velden - TT fields therapie als aanvulling op andere behandelingen zorgen voor uitstekende resultaten bij o.a. borstkanker, hersentumoren en longtumoren, aldus verschillende studies.
- Hyperthermie: Informatie over hyperthermie bij elkaar gezet inclusief rapporten van aantal studies bij o.a. borstkanker, blaaskanker, hyperthermie als aanvulling op bestraling enz..
- HIFU = High Intensity Focused Ultrasound: Bottumoren verwijderen met HIFU = High Intensed Focused Ultrasound naast chemo geeft uitstekende resultaten ten opzichte van alleen chemo of aleen operatie. Artikel update 6 november 2010
- Hormoontherapie om bijwerkingen van overgang te verminderen heeft effect op structuur van hersenen en cognitieve functies blijkt uit placebo gecontroleerde studie
- Hyperbare zuurstof geeft uitstekende resultaten op het herstel van beschadigingen door bestralingen en geeft ook veel pijnverlichting. 76.7 tot 92.6 procent betere kwaliteit van leven
- IMRT - intensiteitsgemoduleerde radiotherapie
- INGN 241 , een nieuw virsuachtig medicijn dat direct in tumor moet worden ingespoten, geeft in fase I studie bij 22 kankerpatiënten 100% resultaat. Artikel geplaatst juni 2005
- Ipilimumab, een immuuntherapeutisch medicijn geeft hoopgevende resultaten op langere overleving bij patienten met een melanoom graad III en IV die al eerder intensief behandeld zijn. FDA keurt ipilimumab goed als medicijn bij gevorderde melanomen
- Lymfklierkanker: reviewstudie van 37 case studies met meerdere vormen van kanker uit een groep van 810 patiënten met lymfklierkanker en hoe bepaalde reguliere aanpak wel of niet werkte en deze studie geeft mooi inzicht in behandeling van lymfklierkanker
- Melanomen: overzicht van artikelen en studies binnen reguliere oncologie. Scroll in linkerkolom voor artikelen. Update 2 april 2010 copy 1
- Metformine: Kankerpatiënten met diabetes (type 2) die metformine gebruiken overleven langer en blijven langer zonder ziekteprogressie dan kankerpatienten plus suikerziekte die geen metaformine gebruiken. Zelfs beter dan kankerpatienten zonder diabetes
- Morfine: Obstipatie bij morfine en andere opioiden als pijnstiller wordt verlicht door Relistor is een officieel goedgekeurd medicijn tegen obstipatie bij o.a. morfinegebruik. Toegevoegd TNO rapport over obstipatie bij sterke pijnmedicatie via morfine
- Nanotechnologie - nanotherapie wordt als toepassing bij medicijnen steeds vaker gebruikt.
- Newcastle Disease Virus - NDV informatie
- NOVO TTF behandeling met electrische pulsen en thuis te gebruiken voor meerdere vormen van kanker
- Pijnbestrijding bij kanker: enkele opties bij elkaar gezet
- PIPAC - verneveling van chemotherapie toegediend via kijkoperatie in de buikholte voor met name buikvliestumoren geeft goede resultaten
- Probiotica - melkzuurbacterien: informatie over wat is probiotica en effecten van probiotica bij o.a. darmkanker en ziekte van Crohn copy 1
- Protonenbestraling
- Provenge als immuuntherapeutisch middel bij gevorderde prostaatkanker geeft significant meer overlevingen indien gegeven samen met dendritische celtherapie. Blijkt uit Fase III studie.
- RFA - Radio Frequency Ablation: een overzicht van recente ontwikkelingen
- Reolysin, een overzicht van recente ontwikkelingen en belangrijke studies met dit virus medicijn
- Sarcomas - Ewing sarcoma: klik hier voor meer artikelen over reguliere ontwikkelingen bij sarcomas en ewing sarcoma
- Scans en ook foto's bij de tandarts veroorzaken honderden nieuwe gevallen van kanker per jaar alleen al in Engeland en duizenden doden door kanker wereldwijd aldus studie aan universiteit van Oxfort
- TACE - Trans Arteriele Chemo Embolisatie en LITT - Laser Induced Interstitual Thermotherapy informatie
- Thalidomide: REVLIMID - lenalidomide studie bij hormoonresistente prostaatkanker wordt gestopt wegens geen resultaat. Bij andere vormen van kanker - CLL en non-Hodgkin gaan studies wel verder met tot nu toe goede resultaten
- Tyrosine-kinase remmers: waarschuwing voor interacties met andere medicijnen die werking van deze klasse van medicijnen kan verminderen of blokkeren stellen Nederlandse onderzoekers in The Lancet
- Ultra Sound = RFA naast TACE geeft hoog significant betere resultaten dan alleen TACE bij levertumoren met een gemiddelde grootte van 10,4 cm. !!!!!, aldus twee gerandomiseerde studies bij totaal 200 patiënten met levertumoren.
- Voeding en voedingstoffen en effect naast reguliere behandelingen: Onder onderzoek en voeding staan vele artikelen en studies die bewijzen dat aanvullende voeding en voedingsuppletie naast reguliere behandelingen vaak de effectiviteit van de behandeling v
- Yttrium-90: Embolisatie met microspheres (Yttrium-90) direct gevolgd door RFA blijkt voor inoperabele levertumoren succesvolle behandeling.
- Zometa - Zoledronic Acid informatie: recente ontwikkelingen en belangrijke studies bij elkaar gezet




Plaats een reactie ...
Reageer op "PIPAC - verneveling van chemotherapie toegediend via kijkoperatie in de buikholte voor met name buikvliestumoren geeft goede resultaten"