Helpt u ons aan 500 donateurs?
22 januari 2018: Bron: EPMA J. 2015; 6: 25. Published online 2015 Dec 21.
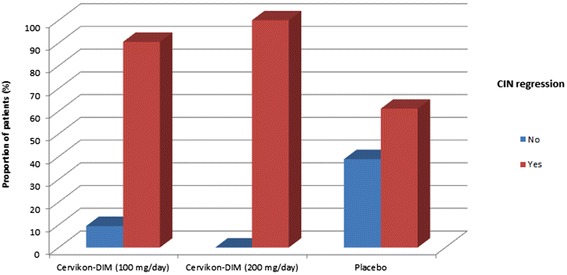
Wanneer vrouwen met intraepithelial neoplasia (CIN) I–II (poliepen), dus met hoog risico op het krijgen van baarmoederhalskanker dagelijks 200 mg per dag DIM - diindolylmethane nemen naast de gebruikeljke medicijnen dan hebben zij weinig tot geen kans op het krijgen van baarmoederhalskanker want binnen 90 tot 180 dagen verdwenen de CIN i en II afwijkingen) volledig. In vergelijking als de DIM dosis 100 mg. per dag was of een placebo waren de CIN afwijkingen verdwenen bij respectievelijk 90 procent en 61 procent voor placebo.
Het gebruik van DIM - diindolylmethaan in de vorm van intravaginale zetpillen kan dus effectief zijn bij patiënten met CIN I-II en gaat niet gepaard met klinisch significante bijwerkingen, aldus de onderzoekers. Deze aanpak zou een betere optie kunnen zijn voor jonge vrouwen met CIN I-II, omdat het hun eventuele kinderwensen beschermd en in tact laat. Aldus de onderzoekers. Het is een kleine fase IIA studie maar wel placebo dubbelblind gerandomiseerd dus een kwalitatief goede studie.
DIM 100 mg capsules zijn overall verkrijgbaar maar doe het aub in overleg met een complementair arts.
Uit het studierapport:
The efficacy of active drug treatment (100 and 200 mg/day) in both treatment groups was significantly higher in comparison with the placebo group, according to the primary efficacy end point (proportion of patients with complete CIN regression after 90–180 days of the study drug treatment).
Het is bekend dat baarmoederhalskanker voornamelijk wordt veroorzaakt door het HPV virus dat meestal begint met intraepithelial neoplasia (CIN) afwijkingen (stadium I, II en III)
Uit het studierapport:
CIN I resolves spontaneously in 50–60 % of cases within 3 years. At the same time, intraepithelial neoplasia develops within 2 years in 15–30 % of women infected with high-risk HPV [6], while about 10–20 % of CIN III cases transform into invasive cervical cancer [7].
In het volledige studierapport: Double-blind randomized placebo-controlled multicenter clinical trial (phase IIa) on diindolylmethane’s efficacy and safety in the treatment of CIN: implications for cervical cancer prevention staat een gedetailleerde beschrijving van de aanpak, maar ook van waarom de onderozkers denken dat DIM zo goed werkt en op welke specifieke genen dit invloed heeft. Maar is voor mij te medisch technisch en is wellciht beter dit met een arts te bespreken. Maar arts-bioloog drs. Engelbert Valstar is over deze studie wel enthousiast.
Hier het abstract van de studie met referentielijst.
the use of diindolylmethane in the form of intravaginal suppositories can be effective in patients with CIN I–II and is not accompanied by clinically significant side effects. This approach could be a better option for young women with CIN I–II as it takes in attention their reproductive plans.
Double-blind randomized placebo-controlled multicenter clinical trial (phase IIa) on diindolylmethane’s efficacy and safety in the treatment of CIN: implications for cervical cancer prevention
Levon Ashrafian, Gennady Sukhikh, Vsevolod Kiselev, Mikhail Paltsev, Vadim Drukh, Igor Kuznetsov, Ekaterina Muyzhnek, Inna Apolikhina, and Evgeniya Andrianova
Abstract
BACKGROUND:
The article presents the results of a clinical trial on the efficacy and safety of a novel pharmaceutical composition in the form of vaginal suppositories containing diindolylmethane in the course of cervical intraepithelial neoplasia (CIN) I-II conservative treatment. It offers an attractive drug therapy for more personalized prevention of cervical cancer.
METHODS:
A total of 78 women of reproductive age were included. This was a multicenter, randomized, placebo-controlled, double-blind, parallel-group trial with efficacy determined by histological evaluation of cervical biopsies. The efficacy of active drug treatment (100 and 200 mg/day) in both treatment groups was significantly higher in comparison with the placebo group, according to the primary efficacy end point (proportion of patients with complete CIN regression after 90-180 days of the study drug treatment).
RESULTS:
The efficacies were 100.0 % (confidence interval (CI) 95 %: 82.35-100.00 %), 90.5 % (CI 95 %: 69.62-98.83 %), and 61.1 % (CI 95 %: 35.75-82.70 %), for the high dose, low does, and placebo, respectively. Adverse events in the placebo group were reported in 22 % of patients (CI 95 %: 7.5-43.7 %); in the first treatment group (100 mg/day), adverse events were reported in 40.0 % of patients (CI 95 %: 21.1-61.3 %); in the second treatment group (200 mg/day), adverse events were reported in 42.0 % of patients (CI 95 %: 22.1-63.4 %). The differences in side effects between treatment groups treated with the active drug and placebo were statistically significant. No serious adverse events were reported in any of the groups.
CONCLUSIONS:
Thus, the use of diindolylmethane in the form of intravaginal suppositories can be effective in patients with CIN I-II and is not accompanied by clinically significant side effects. This approach could be a better option for young women with CIN I-II as it takes in attention their reproductive plans.
TRIAL REGISTRATION:
ID: ChiCTR-INR-15007497 (2 December 2015).
References

Gerelateerde artikelen
- Algemeen: Voeding en voedingstoffen die een preventief effect hebben om kanker: te voorkomen. Een aantal studies en aanbevelingen bij elkaar gezet.
- ALS - amyotrofische laterale sclerose is via een bloedtest 10 jaar eerder te ontdekken voordat symptomen tot klachten gaan leiden
- Alzheimer - dementie is via een bloedtest jaren voordat de ziekte zich openbaart op te sporen en daardoor wellicht ook te voorkomen of uit te stellen voordat de ziekte ernstig wordt.
- Antibiotica speelt mogelijk rol bij ontstaan PDS - Prikkelbare Darm Syndroom.
- Asbest lijkt ook kanker in het strottenhoofd, maagkanker en darmkanker te kunnen veroorzaken.
- Aspirine ter voorkoming van kanker of een recidief van kanker: een overzicht van studies met aspirine copy 1
- Bacterien in de mond - Commensale microbiome en met name Genera Corynebacterium en Kingela spelen grote rol in wel of niet ontwikkelen van mond- en keelkanker. Vooral bij zware rokers en drinkers.
- Diakonessenhuis Utrecht biedt VEGA-Checker aan die bloedwaarden controleert of je lichaam wel alle vitamines en mineralen binnenkrijgt die het nodig heeft.
- DIM - diindolylmethane voorkomt voor 100 procent baarmoederhalskanker (100 procent vs 61 procent bij placebo) bij vrouwen met hoog risico door aanwezigheid van intraepithelial neoplasia (CIN I–II)
- Bloedbiomarker waarden van CRP, LDL cholesterol en lipoproteïne (a), kunnen vrouwen tientallen jaren van tevoren een beeld geven van hun risico op hartziekten
- Bloedtest die methyl meet in cellen kan ruim van te voren voorspellen of iemand borstkanker gaat ontwikkelen. Blijkt uit jarenlang onderzoek. copy 1
- Baarmoederhalskanker veel beter - 60 procent - te voorkomen door DNA test op het HPV virus in vergelijking met uitstrijkje
- Baarmoederkanker is te voorkomen (tot 40 procent) met veel bewegen en stabiel lichaamsgewicht. Te dik geeft meer risico op baarmoederkanker.
- Bewegen: Mensen die te weinig bewegen en zittend hun dagen doorbrengen hadden 82 procent hoger risico om te overlijden aan kanker vergeleken met mensen die veel bewegen en sporten, zelfs na correctie voor leeftijd, geslacht en ziektestatus.
- Bovine Lactoferrin (bLF) stimuleert het immuunsysteem en remt groei darmpoliepen, aldus dubbelblinde gerandomiseerde studie.
- Borstkanker - preventie: een overzicht van belangrijke artikelen en recente studies hoe het risico op borstkanker te verkleinen
- BRCA-1 mutatie verdubbelt risico op baarmoederkanker bij vrouwen met BRCA-1 gen en 26x grotere kans in vergelijking met vrouwen zonder BRCA mutaties
- Cardiovasculaire risicofactoren: Hoe meer cardiovasculaire risicofactoren iemand had als kind en tiener - zoals obesitas, hoge bloeddruk en hoog cholesterolgehalte - hoe lager ze presteerden op geheugen- en denktesten na hun dertigste en 40e.
- Cardiorespiratoire fitheid verbeteren door sporten en bewegen kan ontstaan van prostaatkanker verminderen, blijkt uit Zweedse studie bij circa 50000 mannen
- Chemo en bestraling voor kinderen met kanker vergroot 6 tot 13 keer het risico op krijgen van borstkanker voor hun 40e jaar in vergelijking met bevolkingsrisico.
- Darmbarriere speelt een centrale rol in onze gezondheid. Verstoringen zorgen voor een groter risico op stofwisselingsziektes en speelt ook grote rol in immuniteit.
- Microbioom - Darmflora, een aantal artikelen bij elkaar gezet
- Darmkanker: Een periodieke colonoscopie - inwendig darmonderzoek - vermindert het risico op darmkanker stadium IIB of hoger met 70%.
- Depressie en angst leiden niet tot meer vormen van kanker blijkt uit jarenlang internationaal onderzoek
- Diabetes: Harmine, een natuurlijke alkaloide, blijkt de insuline productie te herstellen bij diabetes patienten en lijkt uitstekende behandeling om diabetes 1 en 2 te genezen
- Diagnostische fouten komen vaak voor in ziekenhuizen. 1 op de 14 patienten krijgt verkeerde diagnose maar is heel vaak te voorkomen.
- Gordelroosvaccin verlaagt risico met meer dan 20 procent op hart- en vaatziekten en blijft actief beschermen tot wel acht jaar na eerste vaccinatie
- Het Kytogeen dieet - koolhydraatarm dieet - strikt volgen kan schadelijk zijn. Lage hoeveelheid vezels en hoog vetgehalte leidt tot onevenwichtige darmflora.
- Hormoontherapie na de menopauze kan risico op kanker verhogen of verkleinen. Oestrogeen verhoogt. Oestrogeen plus Progesteen verkleint.
- HST - Hormoontherapie in en tijdens de overgang veroorzaakt 54 tot 93% kans op galwegziekte, aldus dubbelblinde gerandomiseerde studie bij ruim 14.000 gezonde vrouwen
- Infecties veroorzaken 16 procent van alle vormen van kanker wereldwijd. Leefstijl plus infecties zou voor 35 procent verantwoordelijk zijn aldus grote epidemologische studie in 184 landen
- Leefstijl en voeding in eerste twintig jaar van een mensenleven lijkt bepalend voor risico op krijgen van kanker toont grote Zweedse studie.
- Longkanker: Rauwe knoflook zou de kans op longkanker (44 procent) sterk verminderen. Blijkt uit groot Chinees onderzoek
- Mobiele telefoons geven verhoogd risico op kanker bij kinderen
- Nieuwe richtlijn ‘Overgewicht en obesitas bij volwassenen’ waarbij meetlint de weegschaal vervangt
- NIPT = Niet Invasieve Prenatale celvrije DNA-Test die geen resultaat laat zien of abnormale data betekent vaak (52 procent) dat de moeder beginnende kanker heeft blijkt uit de IDENTIFY studie
- Paddenstoelen: hogere dagelijkse consumptie van paddenstoelen geeft minder risico op krijgen van kanker. Verschil kan oplopen tot 47 procent.
- Parkinson: Aantal patienten met ziekte van Parkinson is schrikbarend gegroeit, vooral door milieuvervuiling als fijnstof en landbouwgif die worden gebruikt in de landbouw en tuinbouw
- PFAS producten lijken groter risico te geven op vormen van hormoongerelateerde kanker bij vrouwen, zoals eierstokkanker maar ook op een melanoom
- Ploegendienst met nachtdiensten vergroot de kans op het ontwikkelen van eierstokkanker
- Probiotica - melkzuurbacteriën kunnen in veel gevallen ziektes voorkomen of zorgen voor herstel van darmflora na chemo of bestraling
- Prostaatkanker: Dieet met groenten, fruit, vis, peulvruchten en volkoren granen vermindert duidelijk de kans op overlijden aan niet uitgezaaide prostaatkanker bij diagnose in vergelijking met westers dieet copy 1
- Psycho stimulerende middelen zoals Ritalin aan kinderen geeft verhoogde kans op krijgen van kanker later. Na 3 maanden ontstond een ernstige chromosoom afwijking. Fase III studie toegevoegd naar effecten van psycho stimulerende middelen op gezondheid
- ReCET - electroporation therapy is een nieuwe endoscopische behandeling en verbetert glykemische controle en stopt behoefte aan medisch toepassen van insuline bij patiënten met diabetes type 2
- Roken is nog schadelijker dan gedacht, maar stoppen met roken kan ook je leven verlengen.
- Screening: Draagbaar ultrasound scanapparaat blijkt uitstekende resultaten te geven bij ontdekken van kwaadaardige tumoren bij mensen met hoog risico op ontwikkelen van borstkanker
- Screeningtesten voor opsporen van kanker zoals voor borstkanker, longkanker, darmkanker en prostaatkanker lijken geen invloed te hebben op uiteindelijke levensduur
- Slaapmiddelen die regelmatig worden gebruikt door volwassenen zouden een 3 keer zo hoog risico geven eerder te sterven en meer kans op kanker in vergelijking met zelden of nooit slaapmiddelen gebruik
- Sociale stress zoals discriminatie en gezinsproblemen, samen met werk- en geldproblemen, kunnen bijdragen aan vroegtijdige veroudering van menselijk immuunsysteem
- Testosteronwaarde is sterk gerelateerd aan botdichtheidsverlies Bijna de helft van de mannen met een laag testosteron had osteopenie of osteoporose.
- Uromune vaccin is bijzonder effectief voor patiënten met recidiverende urineweginfecties (rUTI). 54 procent bleef 9 jaar gevrijwaard van een urineweginfectie zonder gebruik van antibiotica
- Vibratorgebruik kan de bekkengezondheid verbeteren bij vrouwen. Vibratorgebruik verbetert seksuele gezondheid, urine-incontinentie en vulvaire pijn
- Algemene informatie preventie



Plaats een reactie ...
Reageer op "DIM - diindolylmethane voorkomt voor 100 procent baarmoederhalskanker (100 procent vs 61 procent bij placebo) bij vrouwen met hoog risico door aanwezigheid van intraepithelial neoplasia (CIN I–II)"