Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
En als donateur kunt u ook korting krijgen bij verschillende bedrijven:
13 december 2015: Bron: BMC Cancer. 2012; 12: 526. Published online 2012 Nov 16.
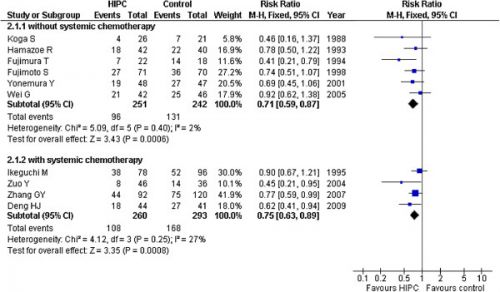
Aanvullend op onderstaand artikel is in 2012 deze meta analyse gepubliceerd die ook aangeeft dat een Hypec operatie (dus met hyperthermie - verwarmde chemo - tijdens de operatie) een betere kans op overleving geeft dan alleen operatie en chemo bij maagkanker. in vergelijking met alleen operatie en / of alleen chemo postoperatief Er moet voor deze behandeling wel goed geselecteerd worden op lichamelijke gesteldheid van de patiënt om ernstige complicaties tijdens de Hypec te voorkomen, maar dat geberut in principe altijd al bij een Hypec operatie. Hier de grafiek van de studies die zijn opgenomen in deze meta analyse en de resultaten daarvan in een grafiek weergegeven
Het volledige studierapport: Benefits of hyperthermic intraperitoneal chemotherapy for patients with serosal invasion in gastric cancer: a meta-analysis of the randomized controlled trials is gratis in te zien. Het abstract hiervan staat onderaan dit artikel
29 april 2005: Bron: Ai Zheng. 2005 Apr;24(4):478-82.
Een combinatiebehandeling van operatie, chemo en inwendige lokale hyperthermie geeft significant meer 2 en 3 jaars overlevingen bij maagkanker t.o.v. alleen operatie met chemo of operatie met alleen gedistilleerd water direct na operatie gegeven. Ook bleek in de groep met hyperthermie als aanvulling significant minder leveruitzaaiïngen op te treden dan in controlegroep. Dit blijkt uit gerandomiseerde studie bij 156 maagkankerpatiënten verdeeld over drie groepen. Groep 1 kreeg combinatie behandeling van operatie en chemo inclusief hyperthermie, groep 2 kreeg chemo direct na operatie en tijdens operatie en groep 3 kreeg gedistilleerd water tijdens en na operatie. Hier de resultaten en conclusie zo goed als letterlijk vertaald in het Nederlands:
RESULTATEN: De 2-jaars overlevingscijfers van de combinatiegroep 1 waren significant beter dan van de controlegroep (88.4% vs. 65.2%, P < 0.05). De 3-jaars overlevingscijfers van groep 1 was significant beter dan van beide andere groepen, (red: al zit er tussen groep 2 en controlegroep opvallend weinig verschil.) (71.1% vs. 50.0% tussen groep 1 en groep 2 , en 71,1% van groep 1 t.o.v. 45,6% van controlegroep. P < 0.05). Het later optreden van leveruitzaaiïngen was vooral in groep 1 maar ook in groep 2 significant lager dan in de controlegroep. (7.7%, en 10.2% vs. 27.3%, P < 0.05).
Conclusie: Een combinatietherapie van intra-operatieve chemo en hyperthermie en snel gegeven postoperatieve chemo kan worden gebruikt om postoperatieve leveruitzaaiïngen te voorkomen.
Achtereenvolgens het abstract van de nieuwste studie en daaronder het abstract van andere studie met zelfde positieve effecten van zelfde aanpak van combinatie van operatie, chemo en hyperthermie gepubliceerd in 2004
Ai Zheng. 2005 Apr;24(4):478-82
[Efficacy of Intraoperative Hypotonic Peritoneal Chemo-hyperthermia Combined with Early Postoperative Intraperitoneal Chemotherapy on Gastric Cancer.]
[Article in Chinese]
Wei G, Fang GE, Bi JW, Shen XJ, Nie MM, Xue XC, Hua JD.
Department of General Surgery, Changhai Hospital, The Second Military Medical University, Shanghai, 200433, P. R. China. wgdr2004@163.com.
BACKGROUND & OBJECTIVE: Abdominal recurrence from exfoliated cancer cells contributes a lot to treatment failure of advanced gastric cancer. Intraperitoneal chemotherapy, which has been proved effective in eliminating exfoliated cancer cells in abdominal cavity, is a hot topic on treatment of gastric cancer. This study was to explore application of combined therapy of intraoperative hypotonic peritoneal chemo-hyperthermia and early postoperative intraperitoneal chemotherapy to gastric cancer.
METHODS: A total of 156 gastric cancer patients were randomized into 3 groups, and underwent the combined therapy (treatment group 1), intraoperative chemotherapy (treatment group 2), and peritoneal lavage with distilled water (control group), respectively.
RESULTS: The 2-year survival rate of treatment group 1 was significantly higher than that of control group (88.4% vs. 65.2%, P < 0.05). The 3-year survival rate of treatment group 1 was significantly higher than those of treatment group 2, and control group (71.1% vs. 50.0%, and 45.6%, P < 0.05). Occurrence of liver metastasis was significantly lower in treatment groups 1 and 2 than in control group (7.7%, and 10.2% vs. 27.3%, P < 0.05).
CONCLUSIONS: Combined therapy of intraoperative hypotonic chemo-hyperthermia and early postoperative intraperitoneal chemotherapy is effective for gastric cancer. Intraperitoneal chemotherapy can be used to prevent postoperative liver metastasis of gastric cancer.
PMID: 15820074 [PubMed - in process]
Zhonghua Zhong Liu Za Zhi. 2004 Apr;26(4):247-9.
[Postoperative intraperitioneal hyperthermic chemoperfusion combined with intravenous chemotherapy for 82 advanced gastric cancer patients]
[Article in Chinese]
Zuo Y, Xu M, Shen D, Lu WD, Lu JF.
First People's Hospital, Zhangjiagang 215600, China.
OBJECTIVE: To evaluate the efficacy of postoperative intraperitoneal hyperthermic chemoperfusion (IHCP) combined with intravenous chemotherapy for advanced gastric cancer.
METHODS: Eighty-two patients with stage II - IV gastric cancer were postoperatively randomized into two groups; 46 patients in treatment group who received IHCP combined with intravenous chemotherapy for three times and 36 patients in control group who received intravenous chemotherapy only for six times. All patients in the two groups received the same chemo-regimen LFAP (CF + 5-Fu + THP or MIT + PDD) 21 - 28 days after operation.
RESULTS: The 1-year survival rate was 98% (45/46) in the treatment group and 94% (34/36) in the control group without any significant difference (P > 0.05). The 3-year survival rate was 83% (38/46) in the treatment group and 61% (22/36) in the control group with significant difference (P < 0.05). Gastrointestinal reaction in the treatment group was significantly decreased compared with in the control group (37% vs 80%, P < 0.01), whereas no statistically significant difference was noted in bone marrow suppression (P > 0.05).
CONCLUSION: Intraperitoneal hyperthermic chemoperfusion combined with intravenous chemotherapy can prolong survival and reduce gastrointestinal side-effect which provides an effective treatment option for advanced gastric cancer.
Randomized Controlled Trial
PMID: 15312391 [PubMed - indexed for MEDLINE]
Our meta-analysis demonstrated that HIPC may improve the overall survival rate for patients who receive resection for advance gastric cancer potentially, and help to prevent peritoneal local recurrence among patients with serosal invasion in gastric cancer.
Benefits of hyperthermic intraperitoneal chemotherapy for patients with serosal invasion in gastric cancer: a meta-analysis of the randomized controlled trials.
Abstract
BACKGROUND:
In this meta-analysis we aimed to determine the effectiveness and safety of hyperthermic intraperitoneal chemotherapy (HIPC) for patients with advanced gastric cancer who underwent gastrectomy.
METHODS:
In accordance with standard meta-analysis procedures, our study included patients who underwent resection for advanced gastric cancer and were randomly allocated to receive either hyperthermic intraperitoneal chemotherapy or control. We searched PubMed (up to November 2011), EMBASE (up to November 2011), Cochrane Database of Systematic Reviews (CDSR), and Cochrane Central Register of Controlled Trials (CCTR) (up to November 2011). Both published and unpublished trials were included in the analysis, and no search restrictions were imposed. There was no language restriction. The results were analyzed using RevMan 5.1 software, which was provided by Cochrane Collaboration.
RESULTS:
There were ten randomized controlled trials included in the analysis. A total of 1062 patients with gastric cancer in these studies were divided into the HIPC group (n = 518) and control group (n = 544). A significant improvement in survival was observed in the HIPC groups compared to the control group in the mitomycin C (MMC) subgroup (RR = 0.75, 95%CI 0.65-0.86; P < 0.00001) and the 5-FU group (RR = 0.69, 95%CI 0.52-0.90; P < 0.00001); the total RR was 0.73 (95%CI 0.64-0.83; P < 0.00001). Our findings indicated that HIPC potentially exhibited a lower peritoneal recurrence rate in the HIPC group compared to the control group (RR = 0.45, 95%CI 0.28-0.72; P = 0.001).
CONCLUSIONS:
Our meta-analysis demonstrated that HIPC may improve the overall survival rate for patients who receive resection for advance gastric cancer potentially, and help to prevent peritoneal local recurrence among patients with serosal invasion in gastric cancer.
- PMID:
- 23153379
- [PubMed - indexed for MEDLINE]
- PMCID:
- PMC3551633
-
References
- Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Neqri E, Malvezzi M, La VC. Recent patterns in gastric cancer: A global overview. Int J Cancer. 2009;125:666–673. doi: 10.1002/ijc.24290. [PubMed] [Cross Ref]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [PubMed] [Cross Ref]
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12(3):354–362. [PMC free article] [PubMed]
- Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374(9688):477–490. doi: 10.1016/S0140-6736(09)60617-6. [PMC free article] [PubMed] [Cross Ref]
- Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL. et al. Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(SICI)1097-0142(20000115)88:2<358::AID-CNCR16>3.0.CO;2-O. [PubMed] [Cross Ref]
- Roviello F, Marrelli D, Manzoni GD, Morqaqni P, Di Leo A, Saraqoni L, De Stefano A. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg. 2003;90:1113–1119. doi: 10.1002/bjs.4164. [PubMed] [Cross Ref]
- Yamada E, Miyaishi S, Nakazato H. The surgical treatment of cancer of the stomach. Int Surg. 1980;65:387–399. [PubMed]
- Yan TD, Black D, Suqarbaker PH, Zhu J, Yonemura Y, Petrou G, Morris DL. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intra-peritoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007;14:2702–2713. doi: 10.1245/s10434-007-9487-4. [PubMed] [Cross Ref]
- Sugarbaker PH, Yu W, Yonemura Y. Gastrectomy, peritonectomy, and perioperative intraperitoneal chemotherapy: The evolution of treatment strategies for advanced gastric cancer. Semin Surg Oncol. 2003;21:233–248. doi: 10.1002/ssu.10042. [PubMed] [Cross Ref]
- Xu DZ, Zhan YQ, Sun XW, Cao SM, Geng QR. Meta-analysis of intraperitoneal chemotherapy for gastric cancer. World J Gastroenterol. 2004;10(18):2727–2730. [PMC free article] [PubMed]
- Shiu MH, Fortner JG. Intraperitoneal hyper-thermic treatment of implanted peritoneal can-cer in rats. Cancer Res. 1980;40:4081–4084. [PubMed]
- Spratt JS, Adcock RA, Muskovin M, Sherrill W, McKeown J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980;40:256–260. [PubMed]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [PubMed] [Cross Ref]
- Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. doi: 10.2307/3001666. [Cross Ref]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [PMC free article] [PubMed] [Cross Ref]
- Koga S, Hamazoe R, Maeta M, Shimizu N, Murakami A, Wakatsuki T. Prophylactic cancer therapy for peritoneal recurrence of gastric by continuous hyperthermic peritoneal perfusion with Mitomycin C. Cancer. 1988;61(2):232–237. doi: 10.1002/1097-0142(19880115)61:2<232::AID-CNCR2820610205>3.0.CO;2-U. [PubMed] [Cross Ref]
- Hamatoe R, Maeta M, Kaibara N. lntraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Cancer. 1994;73(8):2048–2052. doi: 10.1002/1097-0142(19940415)73:8<2048::AID-CNCR2820730806>3.0.CO;2-Q. [PubMed] [Cross Ref]
- Fujimura T, Yonemura Y, Muraoka K. et al. Continuous hyperthermic peritoneal perfusion for the prevention of peritoneal recurrence of gastric cancer: randomized controlled study. Wrold J Surg. 1994;18(1):150–155. doi: 10.1007/BF00348209. [PubMed] [Cross Ref]
- Ikeguch M, Kondou A, Oka A. et al. Effects of continuous hyperthermic peritoneal perfusion on prognosis of gastric cancer with serosal invasion. Eur J Surg. 1995;161(8):581–586. [PubMed]
- Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intra-peritoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1999;85:529–554. doi: 10.1002/(SICI)1097-0142(19990201)85:3<529::AID-CNCR3>3.0.CO;2-9. [PubMed] [Cross Ref]
- Yonemura Y, de Aretxabala X, Fujimura T. et al. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomised controlled study. Hepatogastroenterol. 2001;48:1776–1782. [PubMed]
- Zuo Y, Xu M, Shen D, Lu JF. Postoperative intraperitionealhyperthermic chemoperfusion combined with intravenous chemotherapy for 82 advanced gastric cancer patients. Zhonghua Zhongliu Zazhi. 2004;26:247–249. [PubMed]
- Wei G, Fang GE, Bi JW, Shen XJ, Nie MM, Xue XC, Hua JD. Efficacy of intraoperative hypotonic peritoneal chemo-hyperthermia combined with early postoperative intraperitoneal chemotherapy on gastric cancer. Ai Zheng. 2005;24:478–482. [PubMed]
- Zhang GY, Chen XC, Pan K, Xia LG, Zuo M, Zheng T. Application of hyperthermic intraoperitoneal chemotherapy in patients with gastric cancer. Zhonghua Wei Chang Waike Zazhi. 2007;10(4):362–364. [PubMed]
- Deng HJ, Wei ZG, Zhen L, Li GX, Uang XC, Qing SH. Clinical application of perioperative continuous hyperthermic peritoneal perfusion chemotherapy for gastric cancer. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29(2):295–297. [PubMed]
- Kim JY, Bae HS. A controlled clinical study of serosa-invasive gastric carcinoma patients who underwent surgery plus intraperitoneal hyperthermo-chemo-perfusion (IHCP) Gastric Cancer. 2001;4:27–33. doi: 10.1007/s101200100013. [PubMed] [Cross Ref]
- Sugarbaker PH. Management of Gastric Cancer. Boston: Kluwer Academic Publisher; 1991. pp. 277–284.
- Weisberger AS, Levine B, Storaasli JP. Use of nitrogen mus-tard in treatment of serous effusions of neoplastic origin. JAMA. 1955;159:1704–1707. doi: 10.1001/jama.1955.02960350004002. [PubMed] [Cross Ref]
- Crile G. Jr: Selective destruction of cancers after exposure to heat. Ann Surg. 1962;156:404–407. doi: 10.1097/00000658-196209000-00007. [PMC free article] [PubMed] [Cross Ref]
- MacDonald JS, Malley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM. et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–729. doi: 10.1056/NEJMoa010187. [PubMed] [Cross Ref]
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scrarffe JH, Lofts FJ, Falk SJ, Iveson TJ. et al. Perioperative chemotherapy versus surgery alone for resectable gastro-esophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [PubMed] [Cross Ref]
- Kazuo H, Kanji K, Atsushi I, Yamaquchi A, Nakaqawara G, Umeda S, Kusaka Y. Efficacy of Continuous Hyperthermic Peritoneal Perfusion for the Prophylaxis and Treatment of Peritoneal Metastasis of Advanced Gastric Cancer: Evaluation by Multivariate Regression Analysis. Oncology. 1999;57:106–114. doi: 10.1159/000012016. [PubMed] [Cross Ref]
- Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol. 1998;14(3):254–261. doi: 10.1002/(SICI)1098-2388(199804/05)14:3<254::AID-SSU10>3.0.CO;2-U. [PubMed] [Cross Ref]
Articles from BMC Cancer are provided here courtesy of BioMed Central
Gerelateerde artikelen
- Hyperthermie in combinatie met doelgerichte en immuuntherapeutische behandelingen is veel belovende combinatiebehandeling voor kankerpatienten, aldus artsen van ELMEDIX van de universiteit van Antwerpen copy 1
- Matters studie onderzoekt effecten van hyperthermie bij kankerpatienten met solide tumoren en patienten met alvleesklierkanker. copy 1
- Electro hyperthermie naast bestraling verbetert de 5-jaars overleving met 12 procent (79 vs 91 procent) bij patienten met neuskanker. Hyperthermie gaf ook 17 procent meer complete remissies
- Is de Hyperthermie infra rode sauna voor thuisgebruik (META Chamber Infrared Sauna Dome) een goed en veilig alternatief voor total body hyperthermie?
- Hyperthermie plus hoge dosis vitamine C verbetert overall overleving met 40 procent (9 vs 5 maanden) bij zwaar voorbehandelde met vergevorderde niet-kleincellige longkanker in vergelijking met beste zorg
- Hyperthermie heeft vaak therapeutisch effect in combinatie met chemo en bestraling maar blijkt ook effect te hebben op de kankerstamcellen, ook in combinatie met immuuntherapie, blijkt uit reviewstudie
- Hyperthermie plus voedingsprogramma (kytogeen dieet) plus hyperbare zuurstoftherapie aanvullend op chemo geeft uitstekende resultaten op mediane overall overleving (42 maanden) bij patienten met gevorderde niet-kleincellige longkanker
- Hyperthermie studies in overzichtelijk studierapport samengesteld en gepubliceerd door Nederlandse wetenschappers
- Hyperthermie aanvullend op chemo en bestraling geeft gemiddeld 35 procent betere resultaten dan zonder hyperthermie bewijst reviewstudie met meta analyse van 38 gerandomiseerde studies bij verschillende vormen van kanker
- Algemeen: een vergelijking en beschrijving van verschillende hyperthermie systemen zoals die in Nederland en ons omringende landen worden gebruikt.
- Algemeen: Wetenschappelijk artikel over werking en effect van electro-hyperthermie op basis van gepubliceerde studies. Hyperthermie vandaag: Electrische energie een nieuwe kans bij behandeling van kanker
- Meest geavanceerde kliniek ter wereld voor kinderen met kanker - Universiteit van Dusseldorf - neemt nieuwste hyperthermie apparaat in gebruik voor het behandelen van kinderen met kanker met aanvullend hyperthermie.
- Algemeen: Een heet onderwerp: Hyperthermie als een aanvullende behandeling. Wij vertaalden voor u een groot verklarend artikel over hyperthermie als aanvullende behandeling bij gevorderde kanker.
- Algemeen: hyperthermie heeft in een aantal gerandomiseerde studies bewezen significant het effect van chemokuren en bestralingen te vergroten, aldus artikelen uit the Boston Globe en The American Scientist.
- Algemene informatie hyperthermie: Duits academisch ziekenhuis heeft nieuwste hyperthermie apparatuur in gebruik voor specifieke toepassingen bij kanker in de buik, zoals met name voor eierstokkanker, prostaatkanker, baarmoederhalskanker en darmkanker
- Algemeen: Koorts: vriend of vijand? Koorts speelt een cruciale rol in ons immuunssyteem. Onderdrukken van koorts, vooral bij kinderen, lijkt schadelijk voor ons immuunsyteem. het is beter om koorts uit te laten razen.
- Genezing met warmte: Amerikaans wetenschapper verklaart waarom warmte en acute koorts het immuunsysteem stimuleren in het bestrijden van kanker
- Algemeen: Hyperthermie kan effect van chemo en bestraling versterken en beschadigd DNA sneller herstellen, beweren onderzoekers aan de Erasmus en AMC
- Alvleesklierkanker: Hyperthermie bij alvleesklierkanker, een overzicht van huidige stand van zaken en wetenschappelijk bewijs
- Blaaskanker: diepte hyperthermie naast bestraling en chemo na operatie bij blaaskankerpatienten (T1 en T2) met verhoogd risico op recidief geeft goede resultaten op overall overleving en ziektevrije tijd dan zonder zonder hypert
- Baarmoederkanker: Hyperthermie aanvullend naast bestraling bij gevorderde baarmoederhalskankerpatiënten zorgt ook op langere termijn (12 jaar) voor significant betere resultaten.
- Borstkanker: Hyperthermie bij borstkanker: een overzicht van recente studies en artikelen
- Darmkanker: Hyperthermie naast FOLFOX chemo regiem bij gevorderde darmkanker (stadium II tot IV) verdubbelt 5 jaars overleving t.o.v. controlegroep (30 procent t.o. 15 procent
- Eierstokkanker: Totale lichaams hyperthermie naast op platinum gerelateerde chemokuur bij recidief van eierstokkanker geeft hoopgevende resultaten blijkt uit fase II studie.
- Hersentumoren: Hyperthermie met electromagnetische velden bij recidief van hersentumoren (Astrocytoma graad III en Glioblastoma graad IV) geeft significant langere mediane overleving en significant betere kwaliteit van leven,
- Hoofd en hals tumoren: hyperthermie aanvullend naast bestraling geeft significant, 36 procent, meer complete remissies dan alleen bestraling bij tumoren in het hoofd- en halsgebied
- Kiemcel kanker:Hyperthermie in combinatie met chemotherapie zorgt voor een spectaculaire tumorvermindering en een duidelijke verbetering van de overlevingstijd na 5 jaar van kinderen en jongeren met een recidief van kiemcel kanker of resistent tegen chemo
- Longkanker: Hyperthermie naast chemo bij longkanker graad III/ en V geeft hoopvolle resultaten.
- Maagkanker: Combinatiebehandeling van operatie, chemo en inwendige lokale hyperthermie geeft significant meer 2 en 3 jaars overlevingen bij maagkanker
- Melanomen: Hyperthermie van alleen de lymfklieren rondom laatste recidief lijkt effectief maar succes is afhankelijk van stadium van ziekte blijkt uit recente studie. Artikel geplaatst 5 mei 2010
- Mond- en keelkanker: Hyperthermie met halsband geeft hoopvolle eerste resultaten bij tumoren in hals en mond bij voorbehandelde kankerpatienten met een recidief
- Mond- en keelkanker: Hyperthermie naast bestraling van inoperabele lymfklieren ontstaan vanuit mond- en keelkanker (plaveiselcarcinoom) geeft significant minder bijwerkingen en geeft ook significant betere 5-jaars overleving
- Oppervlakkige tumoren: hyperthermie als aanvulling op bestraling geeft significant betere respons bij oppervlakkige tumoren aldus gerandomiseerde studie.
- Prostaatkanker: 75 jarige man met uitgezaaide prostaatkanker in blaaswand en omliggend weefsel pijnvrij en progressievrij door bestraling en hyperthermie copy 1 copy 1
- Prostaatkanker: Nieuw diep hyperthermie systeem gebruikt naast bestraling bij prostaatkankerpatiënten stadium III en IV zorgt voor 3 jaars overleving van 95 procent t.o.v. 60 procent en tot 73 procent vijf jaars overleving.
- Sarcomas en Hyperthermie: Hyperthermie in combinatie met chemotherapie bij soft sarcomas is succesvol blijkt uit fase III studie. Ziektevrije overlevingstijd wordt verdubbeld en mediane overlevingstijd verlengt met ca. 30 procent.
- Hyperthermie, zowel lokaal als totaal blijkt uitstekende en effectieve aanvullende behandeling bij vele vormen van kanker. Hier een aantal artikelen als overzicht.



Plaats een reactie ...
Reageer op "Maagkanker: Combinatiebehandeling van operatie, chemo en inwendige lokale hyperthermie geeft significant meer 2 en 3 jaars overlevingen bij maagkanker"