9 mei 2017: lees ook dit artikel:
https://kanker-actueel.nl/NL/pembrolizumab-immuuntherapie-met-een-anti-pd-medicijn-geeft-uitstekende-resultaten-bij-recidief-of-progressie-van-voorbehandelde-patienten-met-lymfklierkanker-non-hodgkin.html
2 december 2016: Lees ook dit artikel:
https://kanker-actueel.nl/NL/immuuntherapie-met-extra-gemoduleerde-t-car-cells-geeft-bij-zwaar-voorbehandelde-lymfklierkanker-non-hodgkin-alsnog-uitstekende-resultaten-met-33-procent-complete-remissies.html
2 december 2016: De FDA heeft per 1 december 2016 verdere goedkeuring gegeven aan verder onderzoek naar pembrolizumab (Keytruda) bij klassieke vormen van lymfklierkanker. Zo meldt producent Merck op hun website:
............ the U.S. Food and Drug Administration (FDA) has accepted for review the supplemental Biologics License Application (sBLA) for KEYTRUDA® (pembrolizumab), the company’s anti-PD-1 therapy, for the treatment of patients with refractory classical Hodgkin lymphoma (cHL) or for patients who have relapsed after three or more prior lines of therapy. ..............
“Patients with refractory or relapsed classical Hodgkin lymphoma have limited treatment options,” said Dr. Roger Dansey, senior vice president and therapeutic area head, oncology late-stage development, Merck Research Laboratories. “We believe that the expedited review of this sBLA granted by the FDA is an important step in helping us make KEYTRUDA available as quickly as possible to patients living with this disease.”
The application is seeking approval for KEYTRUDA at a fixed dose of 200 mg administered intravenously every three weeks and is based on data from the KEYNOTE-087 and KEYNOTE-013 trials, which studied patients with refractory cHL or who had relapsed after three or more prior lines of therapy. This is the first application for regulatory approval of KEYTRUDA in a hematologic malignancy.
......lees meer>>>>>>>>
Artikel gaat verder onder deze grafiek
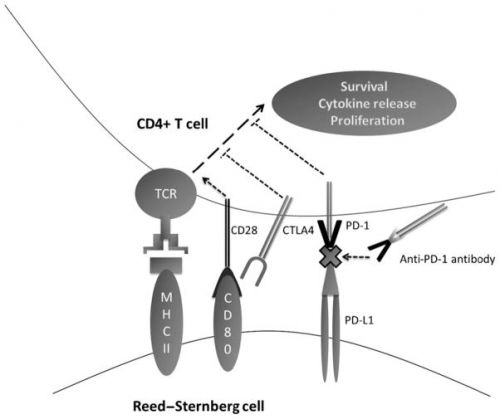
Een interessante reviewstudie over anti-PDF medicijnen bij klassieke lymfklierkanker is deze studie:
met interessanter referentielijst die ik onderaan dit artikel heb gepubliceerd. Scroll ook verder voor meer over pembrolizumab bij klassieke lymfklierkanker
Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting.
En als donateur kunt u ook korting krijgen bij verschillende bedrijven:
https://kanker-actueel.nl/NL/voordelen-van-ops-lidmaatschap-op-een-rijtje-gezet-inclusief-hoe-het-kookboek-en-de-recepten-op-basis-van-uitgangspunten-van-houtsmullerdieet-te-downloaden-enof-in-te-zien.html
16 juli 2016: Bron: Published online before print June 27, 2016, doi: 10.1200/JCO.2016.67.3467 JCO June 27, 2016 JCO673467
Immuuntherapie met pebrozilumab geeft zeer goede resultaten bij zwaarvoorbehandelde patiënten met een klassieke vorm van lymfklierkanker (ziekte van Hodgkin). Van alle deelnemende patienten aan deze veiligheidsstudie (N = 31) had 55% minimaal 4 chemokuren met bv. Brentuximab - Vendotin achter de rug en 71% een stamceltransplantatie maar desondanks toch een recidief.
Uit deze studie met pebrolizumab blijkt dat maar liefst 65% goed reageerde op de anti-PD medicijnen en zelfs 5 patienten (16%) bereikten alsnog een totale remissie = klinisch kankervrij.
Opnieuw het bewijs dat immuuntherapie met een anti-PD medicijn al of niet gecombineerd met bv. T-cell stimulatie voor nagenoeg alle vormen van kanker met solide tumoren de beste aanpak lijkt, zeker als eerdere behandelingen hebben gefaald.
En ik blijf het herhalen: wat zou immuuntherapie met bv. deze anti-PD medicijnen opleveren bij eerste diagnose? Zouden dan ook de bijwerkingen optreden die nu optreden omdat mensen vaak nog een pittige tumorload hebben of verzwakte lichamelijke conditie enz.?
Klik voor een uitstekend gedocumenteerd studierapport naar optredende bijwerkingen bij immuuntherapie met anti-PD medicijnen op het volgende Word document. Immuuntherapie met anti-PD en de bijwerkingen
Studieresultaten:
Aan de studie namen deel 31 patiënten met een recidief of progressie van lymfklierkanker (ziekte van Hodgkin) tijdens of na een behandeling met brentuximab vedotin. Alle patiënten kregen pembrolizumab met een dosis van 10 mg/kg elke twee weken tot zich progressie van de ziekte voordeed of niet meer te dragen bijwerkingen. Overall, 55% van de patiënten had ten minste vier chemokuren gehad en 71% had een recidief gekregen na een autologe stamceltransplantatie.
Bijwerkingen:
De meest voorkomende bijwerkingen gerelateerd aan de behandeling waren: schildklierproblemen hypothyroidism (16%), diarree (16%), misselijkheid (13%), en lichte longontstekingen - pneumonitis (10%).
Graad 3 bijwerkingen gerelateerd aan de behandeling kwamen voor bij 5 patiënten(16%) en bestond uit colitis, verhoogde leverwaarden - ALAT - alanine transaminase en ASAT - aspartate transaminase, nephrotic - niersyndroom, opgezwollen en pijnlijke gewrichten, rugpijn en okselpijn.
Er waren geen graad 4 bijwerkingen en ook overleden er geen mensen tijdens of aan de behandeling. Twee patiënten onderbraken de behandeling tijdens de studieduur wegens een graad 2 longontsteking en graad 3 niersyndroom.
Respons en klinische resultaten:
Bij 20 patiënten (65%), werd een goede respons gezien waaronder een complete remissie bij 5 patiënten (16%). De respons duurde minimaal 24 weken bij 70 procent van de patiënten die een respons lieten zien. Na een mediane follow-up van 17 maanden varieerde de duur van de respons van 0.14+ weken tot 74+ weken. Progressie-vrije overleving was 69% op 24 weken en 46% op 1 jaar.
Hoge expressie van de PD-L1 (programmed cell death ligand 1) en PD-L2 (programmed cell death ligand 2); uitbreiding van door de behandeling veroorzaakte T cellen en natural killer cellen; en activatie van interferon-γ, T-cell receptor en uitbreidende immuun gerelateerde zogeheten 'signaling pathways' werden gezien in een biomarker analyse.
Conclusie:
De onderzoekers concluderen: “Pembrolizumab wordt geassocieerd aan een goed veiligheidsprofiel. Een behandeling met Pembrolizumab veroorzaakte uitstekende klinische resultaten bij patienten met zwaarvoorbehandelde lymfklierkanker - ziekte van Hodgkin en verdere studies worden aanbevolen."
Het volledige studierapport: Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure is gratis in te zien.
Hier ook het abstract van deze studie:
pembrolizumab (Keytruda) is active in patients with disease progressing on or after brentuximab vedotin (Adcetris) treatment and gives even 16 procent complete remissions in heavily pretreated patients with relapsed or refractory Hodgkin lymphoma
Published online before print June 27, 2016, doi: 10.1200/JCO.2016.67.3467 JCO June 27, 2016 JCO673467
2016 by American Society of Clinical Oncology
Programmed Death-1 Blockade With Pembrolizumab in Patients With Classical Hodgkin Lymphoma After Brentuximab Vedotin Failure
- Philippe Armand,
- Margaret A. Shipp,
- Vincent Ribrag,
- Jean-Marie Michot,
- Pier Luigi Zinzani,
- John Kuruvilla,
- Ellen S. Snyder,
- Alejandro D. Ricart,
- Arun Balakumaran,
- Shelonitda Rose and
- Craig H. Moskowitz⇑
+ Author Affiliations
- Philippe Armand and Margaret A. Shipp, Dana-Farber Cancer Institute, Boston, MA; Vincent Ribrag and Jean-Marie Michot, Institut Gustave Roussy, Villejuif, France; Pier Luigi Zinzani, Institute of Hematology Seràgnoli, University of Bologna, Bologna, Italy; John Kuruvilla, Princess Margaret Cancer Centre and University of Toronto, Toronto, Ontario, Canada; Ellen S. Snyder, Alejandro D. Ricart, Arun Balakumaran, and Shelonitda Rose, Merck, Kenilworth, NJ; and Craig H. Moskowitz, Memorial Sloan Kettering Cancer Center, New York, NY.
- Corresponding author: Craig H. Moskowitz, MD, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: moskowic@mskcc.org.
-
Presented at the American Society of Hematology 56th Annual Meeting and Exposition, San Francisco, CA, December 6-9, 2014; and American Society of Hematology 57th Annual Meeting and Exposition, Orlando, FL, December 5-8, 2015.
Abstract
Purpose Classical Hodgkin lymphoma (HL) frequently exhibits genetic alterations leading to overexpression of the programmed death-1 (PD-1) ligands, suggesting a possible vulnerability to PD-1 blockade. The phase Ib study KEYNOTE-013 (NCT01953692) tested the safety and efficacy of the anti–PD-1 antibody pembrolizumab in patients with hematologic malignancies. Based on its genetics, HL was included as an independent cohort.
Methods We enrolled patients with relapsed or refractory HL whose disease progressed on or after treatment with brentuximab vedotin. Patients received pembrolizumab, 10 mg/kg every 2 weeks, until disease progression occurred. Response to treatment was assessed at week 12 and every 8 weeks thereafter. Principal end points were safety and complete remission (CR) rate.
Results Thirty-one patients were enrolled; 55% had more than four lines of prior therapy, and 71% had relapsed after autologous stem cell transplantation. Five patients (16%) experienced grade 3 drug-related adverse events (AEs); there were no grade 4 AEs or deaths related to treatment. The CR rate was 16% (90% CI, 7% to 31%). In addition, 48% of patients achieved a partial remission, for an overall response rate of 65% (90% CI, 48% to 79%). Most of the responses (70%) lasted longer than 24 weeks (range, 0.14+ to 74+ weeks), with a median follow-up of 17 months. The progression-free survival rate was 69% at 24 weeks and 46% at 52 weeks. Biomarker analyses demonstrated a high prevalence of PD-L1 and PD-L2 expression, treatment-induced expansion of T cells and natural killer cells, and activation of interferon-γ, T-cell receptor, and expanded immune-related signaling pathways.
Conclusions Pembrolizumab was associated with a favorable safety profile. Pembrolizumab treatment induced favorable responses in a heavily pretreated patient cohort, justifying further studies.
referentielijst bij pembrolizumab bij lymfklierkanker
Source:
Published in final edited form as:
PMCID: PMC4987237
NIHMSID: NIHMS805054
Pembrolizumab in classical Hodgkin’s lymphoma
References
1.
Küppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2009;9:15–27. [PubMed]2. Swerdlow S. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4. Geneva, Switzerland: WHO Press; 2008.
3.
Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 Trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21:3601–8. [PubMed]4.
Fermé C, Eghbali H, Meerwaldt JH, et al. Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N Engl J Med. 2007;357:1916–27. [PubMed]5.
Gordon LI, Hong F, Fisher RI, et al. Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496) J Clin Oncol. 2013;31:684–91. [PMC free article] [PubMed]6.
Borchmann P, Haverkamp H, Diehl V, et al. Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage Hodgkin’s lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group. J Clin Oncol. 2011;29:4234–42. [PubMed]7.
Horning SJ, Chao NJ, Negrin RS, Hoppe RT, Long GD, Hu WW, Wong RM, Brown BW, Blume KG. High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin’s disease: analysis of the Stanford University results and prognostic indices. Blood. 1997;89:801–13. [PubMed]8.
Lavoie JC, Connors JM, Phillips GL, et al. High-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long-term outcome in the first 100 patients treated in Vancouver. Blood. 2005;106:1473–8. [PubMed]9.
Gerrie AS, Power MM, Shepherd JD, Savage KJ, Sehn LH, Connors JM. Chemoresistance can be overcome with high-dose chemotherapy and autologous stem-cell transplantation for relapsed and refractory Hodgkin lymphoma. Ann Oncol. 2014;25:2218–23. [PubMed]10.
Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, Chopra R, Milligan D, Hudson GV. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341:1051–4. [PubMed]11.
Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;15:2065–71. [PubMed]12.
Kewalramani T, Nimer SD, Zelenetz AD, Malhotra S, Qin J, Yahalom J, Moskowitz CH. Progressive disease following autologous transplantation in patients with chemosensitive relapsed or primary refractory Hodgkin’s disease or aggressive non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2003;32:673–9. [PubMed]13.
Thomson KJ, Peggs KS, Smith P, et al. Superiority of reduced-intensity allogeneic transplantation over conventional treatment for relapse of Hodgkin’s lymphoma following autologous stem cell transplantation. Bone Marrow Transplant. 2008;41:765–70. [PubMed]14.
Sarina B, Castagna L, Farina L, et al. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: a retrospective study based on the time of HLA typing and donor availability. Blood. 2010;115:3671–7. [PubMed]15.
Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–52. [PMC free article] [PubMed]16. Stavrik SGG, Boumendil A, Thomson K, et al. Myeloablative versus reduced intensity allogeneic stem cell transplantation in relapsed Hodgkin’s lymphoma in recent years. A retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2014 abstract 2562, American Society of Hematology Meeting.
17.
Fehniger TA, Larson S, Trinkaus K, et al. A phase 2 multi-center study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood. 2011;118:5119–25. [PMC free article] [PubMed]18.
Buglio D, Younes A. Histone deacetylase inhibitors in Hodgkin lymphoma. Invest New Drugs. 2010;28(Suppl 1):21–7. [PMC free article] [PubMed]19.
Johnston PB, Inwards DJ, Colgan JP, et al. A Phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010;85:320–4. [PMC free article] [PubMed]20.
Josting A, Nogová L, Franklin J, et al. Salvage radiotherapy in patients with relapsed and refractory Hodgkin’s lymphoma: a retrospective analysis from the German Hodgkin Lymphoma Study Group. J Clin Oncol. 2005;23:1522–9. [PubMed]21.
Santoro A, Bredenfeld H, Devizzi L, et al. Gemcitabine in the treatment of refractory Hodgkin’s disease: results of a multi-center phase II study. J Clin Oncol. 2000;18:2615–9. [PubMed]22.
Little R, Wittes RE, Longo DL, Wilson WH. Vinblastine for recurrent Hodgkin’s disease following autologous bone marrow transplant. J Clin Oncol. 1998;16:584–8. [PubMed]23.
Rule S, Tighe M, Davies S, Johnson S. Vinorelbine in the treatment of lymphoma. Hematol Oncol. 1998;16:101–5. [PubMed]24.
Gopal AK, Press OW, Shustov AR, et al. Efficacy and safety of gemcitabine (G), carboplatin (C), dexamethasone (D), and rituximab (R) in patients with relapsed/refractory lymphoma: a prospective multi-center phase II study of by the Puget Sound Oncology Consortium (PSOC) Leuk Lymphoma. 2010;51:1523–9. [PMC free article] [PubMed]25.
Aparicio J, Segura A, Garcerá S, Oltra A, Santaballa A, Yuste A, Pastor M. ESHAP is an active regimen for relapsing Hodgkin’s disease. Ann Oncol. 1999;10:593–5. [PubMed]26.
Bartlett NL, Niedzwiecki D, Johnson JL, Friedberg JW, Johnson KB, van Besien K, Zelenetz AD, Cheson BD, Canellos GP. Cancer Leukemia Group B. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin’s lymphoma: CALGB 59804. Ann Oncol. 2007;18:1071–9. [PubMed]27.
Wheeler C, Khurshid A, Ibrahim J, Elias A, Mauch P, Ault K, Antin J. Incidence of post transplant myelodysplasia/acute leukemia in non-Hodgkin’s lymphoma patients compared with Hodgkin’s disease patients undergoing autologous transplantation following cyclophosphamide, carmustine, and etoposide (CBV) Leuk Lymphoma. 2001;40:499–509. [PubMed]28.
Alinari L, Blum KA. How I treat relapsed classical Hodgkin lymphoma after autologous stem cell transplant. Blood. 2015;127:287–295. [PubMed]29.
Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30:2183–9. [PMC free article] [PubMed]30.
Gopal AK, Chen R, Smith SE, et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:1236–43. [PMC free article] [PubMed]31.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. [PubMed]32.
Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24. 1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–77. [PMC free article] [PubMed]33.
Green MR, Rodig S, Juszczynski P, Ouyang J, Sinha P, O’Donnell E, Neuberg D, Shipp MA. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18:1611–8. [PMC free article] [PubMed]34.
Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–9. [PMC free article] [PubMed]35.
Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. [PubMed]36. Lesokhin AM, Ansell SM, Armand P, et al. Preliminary Results of a Phase I Study of Nivolumab (BMS-936558) in Patients with Relapsed or Refractory Lymphoid Malignancies. Blood. 2014 abstract 291, American Society of Hematology Meeting.
37.
Armand P. Immune checkpoint blockade in hematologic malignancies. Blood. 2015;28:3393–400. [PubMed]38. Zinzani PL, Ribrag V, Moskowitz CH, et al. Phase 1b Study of PD-1 Blockade with Pembrolizumab in Patients with Relapsed/Refractory Primary Mediastinal Large B-Cell Lymphoma (PMBCL) Blood. 2015 abstract 3986, American Society of Hematology Meeting.
39.
Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti–PD-1) in melanoma. N Engl J Med. 2013;11:134–44. [PMC free article] [PubMed]40.
Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373:1627–39. [PubMed]41.
Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. [PubMed]42.
Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32. [PubMed]43.
Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372:2018–28. [PubMed]44.
Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. [PubMed]45. Ansell SM, Armand P, Timmerman J, et al. Nivolumab in Patients (Pts) with Relapsed or Refractory Classical Hodgkin Lymphoma (R/R cHL): Clinical Outcomes from Extended Follow-up of a Phase 1 Study (CA209-039) Blood. 2015 abstract 583, American Society of Hematology Meeting.
46. Herbaux C, Gauthier J, Brice P, et al. Nivolumab Is Effective and Reasonably Safe in Relapsed or Refractory Hodgkin’s Lymphoma after Allogeneic Hematopoietic Cell Transplantation: A Study from the Lysa and SFGM-TC. Blood. 2015 abstract 3979, American Society of Hematology Meeting.
47. Moskowitz CH, Ribrag V, Michot J-M, et al. PD-1 Blockade with the Monoclonal Antibody Pembrolizumab (MK-3475) in Patients with Classical Hodgkin Lymphoma after Brentuximab Vedotin Failure: Preliminary Results from a Phase 1b Study (KEYNOTE-013) Blood. 2014 abstract 290, American Society of Hematology Meeting.
48.
Armand P, Shipp MA, Ribrag V, et al. PD-1 Blockade with Pembrolizumab in Patients with Classical Hodgkin Lymphoma after Brentuximab Vedotin Failure: Safety, Efficacy, and Biomarker Assessment. Blood. 2015 abstract 584, American Society of Hematology Meeting. [PubMed]49.
Kwong Y-L, Lopes D, Khong P-L. Low-dose pembrolizumab induced remission in patients with refractory classical Hodgkin lymphoma. Br J Haematol. 2016 Epub ahead of print. [PubMed]50.
Yared JA, Hardy N, Singh Z, et al. Major clinical response to nivolumab in relapsed/refractory Hodgkin lymphoma after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016 Epub ahead of print. [PubMed]51.
Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–42. [PMC free article] [PubMed]52.
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. [PMC free article] [PubMed]53.
Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. [PMC free article] [PubMed]54.
Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–53. [PMC free article] [PubMed]55.
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–7. [PMC free article] [PubMed]56.
Radhakrishnan S, Nguyen LT, Ciric B, Flies D, Van Keulen VP, Tamada K, Chen L, Rodriguez M, Pease LR. Immunotherapeutic potential of B7-DC (PD-L2) cross-linking antibody in conferring antitumor immunity. Cancer Res. 2004;64:4965–72. [PubMed]57.
Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–22. [PMC free article] [PubMed]58.
Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. [PMC free article] [PubMed]59.
Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin’s lymphoma: increasing evidence of the importance of the microenvironment. J Clin Oncol. 2011;29:1812–26. [PubMed]60.
Scapin G, Yang X, Prosise WW, McCoy M, Reichert P, Johnston JM, Kashi RS, Strickland C. Structure of full-length human anti-PD1 therapeutic IgG4 antibody pembrolizumab. Nat Struct Mol Biol. 2015;22:953–8. [PubMed]61.
Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764–82. [PMC free article] [PubMed]62. PD-1 immune checkpoint inhibitors show promising activity in relapsed/refractory Hodgkin lymphoma. Key lymphoma studies from ASH 2015. Oncologist. 2016 21:Epub ahead of print.
63.
Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol. 2014;15:1311–8. [PMC free article] [PubMed]64.
Fernández-Vega I, Quirós LM, Santos-Juanes J, Pane-Foix M, Marafioti T. Bruton’s tyrosine kinase (Btk) is a useful marker for Hodgkin and B cell non-Hodgkin lymphoma. Virchows Arch Int J Pathol. 2015;466:229–35. [PubMed]65.
Martín P, Salas C, Provencio M, Abraira V, Bellas C. Heterogeneous expression of Src tyrosine kinases Lyn, Fyn and Syk in classical Hodgkin lymphoma: prognostic implications. Leuk Lymphoma. 2011;52:2162–8. [PubMed]66.
Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107:13075–80. [PMC free article] [PubMed]67.
Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539–49. [PMC free article] [PubMed]68.
Hamadani M, Balasubramanian S, Hari PN. Ibrutinib in refractory classic Hodgkin’s lymphoma. N Engl J Med. 2015;373:1381–2. [PubMed]69. Merryman R, Kim H, Zinzani PL, et al. Safety and Efficacy of Allogeneic Hematopoetic Stem Cell Transplant (HSCT) after Treatment with Programmed Cell Death 1 (PD-1) Inhibitors. Blood. 2015 abstract 2018, American Society of Hematology Meeting.
70.
Jefferis R. Isotype and glycoform selection for antibody therapeutics. Arch Biochem Biophys. 2012;526:159–66. [PubMed]71. Wang H, Cheng F, Cheng J, et al. JQ1, a Selective Bromodomain Inhibitor, Augment the Immunogenicity of Mantle Cell Lymphoma By Influencing the Expression of PD-L1. Blood. 2015 abstract 822, American Society of Hematology Meeting.
72.
Yang H, Bueso-Ramos C, DiNardo C, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28:1280–8. [PMC free article] [PubMed]73.
Oki Y, Buglio D, Zhang J, et al. Immune regulatory effects of panobinostat in patients with Hodgkin lymphoma through modulation of serum cytokine levels and T-cell PD1 expression. Blood Cancer J. 2014;4:e236. [PMC free article] [PubMed]
lymfklierkanker, melanomen, non-Hodgkin, immuuntherapie, klinisch kankervrij, Bernhard van Vollenhoven, anti-PD medicijnen, immuuntherpaie, pebrolizumab
Gerelateerde artikelen
- Tafasitamab - Monjuvi in combinatie met lenalidomide - Revlimid en R-CHOP geeft betere ziekteprogressievrije overleving in vergelijking met alleen R-CHOP bij patiënten met lymfklierkanker type diffuus grootcellig B-cellymfoom (Non-Hodgkin)
- loncastuximab tesirine en glofitamab combinatie geeft uitstekende resultaten met 89 procent ziektecontrole en 77 procent complete remissies bij patienten met uitgezaaide gevorderde B-cel non-Hodgkin-lymfoom
- CAR-T celtherapie - Axicabtagene ciloleucel geeft veel betere ziektevrije overleving in vergelijking met de standaardbehandeling van tweedelijnstherapie bij patiënten met recidief, ziekteprogressie van lymfklierkanker type diffuus grootcellig B-cellymfoom
- Immuuntherapie met nivolumab naast standaard chemo als eerstelijns behandeling zorgt voor betere resultaten en minder bijwerkingen bij patienten met klassieke ziekte van Hodgkin stadium III en IV in vergelijking met brentuximab vedotin
- Immuuntherapie met CAR-T celtherapie (isocabtagene maraleucel (liso-cel)) geeft meer complete remissies en betere overall overleving dan chemo + stamceltransplantatie als tweedelijnsbehandeling bij patienten met grootcellig B-cel lymfoom
- Autologe stamceltransplantatie plus anti-CD30 CAR-T celtherapie geeft 5 duurzame complete remissies bij 6 deelnemende patienten met lymfklierkanker die al paar keer een recidief hadden gehad ondanks chemokuren.
- Autologe stamceltransplantatie na immuuntherapie met anti-PD medicijnen geeft zeer goede resultaten voor gevorderde lymfklierkanker - Hodgkin lymfoom zelfs bij zwaar voorbehandelde patiënten
- Glofitamab, een antibody medicijn, alleen en in combinatie met obinutuzumab geeft nog goede respons (36 procdent) bij zwaar voorbehandelde patienten met recidief of refractair B-cel non-hodgkin-lymfoom.
- Mosunetuzumab, een bispecifiek monoklonaal antilichaam, geeft uitstekende resultaten (CR bij 60 procent) bij patienten met een Folliculair lymfoom waarbij eerdere behandelingen faalden.
- Pembrolizumab geeft langdurige complete en gedeeltelijke remissies (4 jaar en langer) bij zwaar voorbehandelde patiënten met klassiek Hodgkin-lymfoom na falen van brentuximab vedotin
- Immuuntherapie met Nivolumab solo of naast chemo (N-AVD) gevolgd door radiotherapie bij patiënten met hoog risico van lymfklierkanker als eerstelijns behandeling geeft uitstekende resultaten op korte termijn
- Bendamustine plus Rituximab gevolgd door behandelingen met 90-Yttrium plus 4x Ibritumomab Tiuxetan voor onbehandelde Folliculaire Lymfomen geeft betere overall overleving en langere duurzame ziektevrije tijd copy 1
- Immuuntherapie met Camrelizumab, een anti-PD medicijn geeft hele goede resultaten met complete en gedeeltelijke remissies bij patienten met recidief of ziekteprogressie van klassieke lymfklierkanker
- Immuuntherapie met gemanipuleerde T-cellen - CAR-T celtherapie ( tisagenlecleucel ) geeft spectaculair goede resultaten bij patienten met gevorderde lymfklierkanker - non-Hodgkin (B-lymfomen) copy 1
- Lenalidomide naast rituximab verbetert mediane progressievrije overleving met 25 maanden (39 vs 14 maanden) bij patienten met recidief van indolente lymfomen - lymfklierkanker, in vergelijking met alleen rituximab
- Ivo Visser uitbehandeld voor zeldzame vorm van lymfklierkanker komt alsnog in total remissie (kankervrij) met T-CAR cel immuuntherapie
- Immuuntherapie met extra gemoduleerde T-car cells geeft bij zwaar voorbehandelde lymfklierkanker non-Hodgkin alsnog uitstekende resultaten met 33 procent complete remissies
- Lymfklierkanker en leukemie kennen verschillende vormen en stadia. Hier een recente studie van de belangrijkste behandelingsopties, vooral met vormen van immuuntherapie
- Obinutuzumab aanvullend op standaard chemokuren, verlengt ziektevrije overall overleving (plus 34 procent) van indolente Non Hodgkin Lymphomen stadium III / IV in vergelijking met standaard chemo plus Rituximab.
- Pembrolizumab, immuuntherapie met een anti-PD medicijn, geeft uitstekende resultaten bij recidief of progressie van voorbehandelde patienten met lymfklierkanker, non-Hodgkin
- Pembrolizumab geeft zeer goede resultaten (65 procent respons) bij klassieke lymfklierkanker (Hodgkin) bij zwaar voorbehandelde patienten en na falen van o.a. brentuximab plus vedotin
- Nivolumab - Opdivo geeft extreem goede resultaten - 87 procent respons - bij zwaar voorbehandelde patienten met lymfklierkanker
- Genentest met 7 nieuwe genmutaties voorspelt of immuuntherapie met Rituximab aanvullend op CHOP kuren zal slagen of falen bij non-Hodgkin - folluculaire lymfoma copy 1
- Brentuximab Vedotin (SGN-35) blijkt succesvolle aanpak voor gevorderde lymfklierkanker, non-hodgkin lymfomen met CD30 positieve expressie.
- BiovaxID, een vaccin, geeft significant langere ziektevrije tijd bij lymfklierkanker (non-Hodgkin) blijkt uit fase III studie.
- Immuuntherapie bij lymfklierkanker: BiovaxID(TM), een Vaccinatie - immuuntherapie bij non-Hodgkin geeft goede resultaten uit fase I en II en fase III trials.
- Immuuntherapie bij lymfklierkanker: Non-Hodgkin en vaccinatie met BiovaxID is succesvolle en veelbelovende aanpak.
- Pentostatin - Nipent lijkt effectief middel om afstoting tegen te gaan bij immuuntherapie en stamceltransplanties bij verschillende vormen van kanker waaronder lymfklierkanker en leukemie
- Immuuntherapie bij lymfklierkanker: een overzicht van recente ontwikkelingen





Plaats een reactie ...
Reageer op "Pembrolizumab geeft zeer goede resultaten (65 procent respons) bij klassieke lymfklierkanker (Hodgkin) bij zwaar voorbehandelde patienten en na falen van o.a. brentuximab plus vedotin"