Uit twee gerandomiseerde studies, de fase II studie PEGASUS en de fase III studie DYNAMIC, blijkt dat een negatief resultaat op aanwezigheid van kankercellen bij patiënten met darmkanker stadium III verkregen via een ctDNA test = circulerend tumor-DNA bloedtest geen verschil maakt met standaard chemotherapie als behandeling.
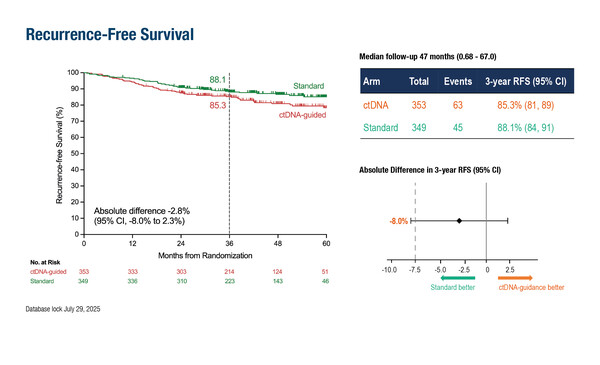
Uit de fase III studie DYNAMIC blijkt dat na een mediane follow-up van 47 maanden de 3-jaars recidiefvrije overleving als resultaat te geven 85,3% in de ctDNA-geleide groep versus 88,1% in de standaard chemogroep (absoluut verschil -2,8%; 95%-betrouwbaarheidsinterval -8,0-2,3), waarmee de ondergrens van -7,5% werd overschreden.
Uit de fase II studie PEGASUS werd de aanwezigheid versus afwezigheid van ctDNA geassocieerd met een slechtere ziektevrije overleving op 3 jaars meting: respectievelijk 58,4% versus 82,8%; hazard ratio 2,70; p=0,0036) en algehele overleving (79,7% versus 93,8%; HR 3,11; p=0,0383) bij een mediane follow-up van 42 maanden. Waarbij aangetekend dat in deze analyse slechts 100 patiënten die een ctDNA hadden gekregen versus slechts 34 patiënten die geen ctDNA-test hadden gehad. Bovendien en dat lijkt cruciaal bij een negatieve uitslag van de ctDNA test, dus dat er geen kankercellen werden gevonden na de operatie leidde tot een vermindering van de chemotherapie, zowel in intensiteit als in tijd. Terwijl bij darmkanker stadium III al uitzaaiingen zijn gevonden in de lymfklieren in de nabijheid van de primaire tumor. En wellicht de operatie niet alle kankercellen heeft kunnen weghalen of waren er al kankercellen hoewel niet aantoonbaar elders in andere lymfklieren en/of organen. Zie uitleg daarover verder in dit artikel.
Grafische weergave van de resultaten uit de fase III studie DYNAMIC: (tekst gaat verder onder deze grafiek)
Waarom deze resultaten kritisch moeten worden bekeken blijkt uit deze korte uitleg:
In de fase III studie DYNAMIC veranderden de oncologen in de klinische parktijk van de ziekenhuizen het geselecteerde chemotherapie behandelingsschema na de operatie voorafgaand aan indeling in de twee groepen naar ctDNA geïnformeerd of standaardbeleid.
Bij ctDNA-geïnformeerd beleid leidde een ctDNA-negatief resultaat 5-6 weken na de operatie met de SaferSeqS-gerichte CRC-paneltest tot een geleidelijk afbouwend behandelingsplan van:
- 6 maanden monotherapie met fluoropyrimidine naar geen chemotherapie of 3 maanden fluoropyrimidine;
- van 3 maanden oxaliplatine plus fluoropyrimidine naar 3-6 maanden fluoropyrimidine;
- of van 6 maanden oxaliplatine plus fluoropyrimidine naar 3 maanden oxaliplatine plus fluoropyrimidine of 6 maanden fluoropyrimidine).
Hieronder achtereenvolgens het abstract van de fase III studie DYNAMIC. Daaronder het abstract van de fase II studie PEGASUS.
723O - Post-surgical liquid biopsy-guided treatment of stage III and high-risk stage II colon cancer patients: Final results of the PEGASUS trial
- Silvia Marsoni (Milan, Italy, (TO))
- Silvia Marsoni (Milan, Italy, (TO))
- Michele Prisciandaro (Milan, Italy)
- Noelia Tarazona Llavero (Valencia, Spain, Valencia)
- Clara Montagut (Barcelona, Spain)
- Andrea Sartore Bianchi (Milan, Italy)
- Maria Giulia Zampino (Milan, Italy)
- Francisco Javier Ros Montana (Barcelona, Spain)
- Cristina Santos Vivas (L'Hospitalet de Llobregat, Spain)
- Mario Mandalà (Perugia, Italy)
- Stefano Tamberi (Ravenna, Italy, Ravenna)
- Maria Stefania Sciallero (Genova, Italy)
- Susana Muñoz (Barcelona, Spain)
- Paolo Luraghi (Milan, Italy)
- Luca Lazzari (Milan, Italy)
- Eliana Rulli (Milan, Italy)
- Valter Torri (Milan, Italy)
- Elena Elez Fernandez (Barcelona, Spain)
- Alberto Bardelli (Torino, Italy, (TO))
- Salvatore Siena (Milan, Italy)
- Sara Lonardi (Padua, Italy)
Background
Prognostic impact of circulating tumor DNA (ctDNA) positivity on colon cancer disease relapse after radical resection and adjuvant chemotherapy has been previously demonstrated. Here we present the final analysis on the primary endpoint of the PEGASUS trial, designed to investigate the feasibility of using liquid biopsy (LB) to guide the post-surgical and post-adjuvant clinical management of Stage III and T4N0 Stage II colon cancer patients (pts).
Methods
LB was performed using Guardant Reveal assay (v L1.2). In the post-surgical phase, LB+ pts received 3 months of CAPOX and LB- pts 6 months of CAPE. To mitigate the risk of false negatives, after one cycle of CAPE a second LB was performed and if positive the treatment was escalated to CAPOX. At the end of the adjuvant treatment, LB was repeated: CAPOX-treated patients were switched to FOLFIRI if LB+ or de-escalated to CAPE if LB-; CAPE-treated patients received CAPOX if LB+ or standard follow-up if LB-. Primary objective was feasibility of the LB-based strategy, defined as the rate of pts free of disease at 2 years among those with two consecutive post-surgical LB– results. With one-sided α=0.05 and 80% power, <14 relapses among 134 LB- pts were required to exclude H0=85% in favor of H1=92%. A matched comparison with TOSCA Trial pts was planned as a secondary endpoint.
Results
From July 2020 to July 2022, 135 pts (100 LB-, 35 LB+) were included from 11 clinical cancer centers in Italy and Spain. At a median follow-up of 33.5 months, 12 relapses occurred among LB– pts, yielding a 2-year NED rate of 88% (90% CI, 81–93). The predefined threshold was not met, but the point estimate exceeded 85% and the confidence interval extended beyond 92%, indicating promise but statistical uncertainty. Within the 35 LB+ pts, 13 (37%) relapses were observed. 2-years time-to-relapse rate was 87.6% in LB- and 61.5% in LB+ (HR 3.22, 95% CI 1.20–7.96, p=0.0013).
Conclusions
PEGASUS showed the operational feasibility of ctDNA-guided treatment in stage III and high-risk stage II resected colon cancer. This approach has the potential to reduce unnecessary toxicity by tailoring adjuvant therapy to molecular risk. A historical comparative analysis with pts from the TOSCA trial is ongoing as per-protocol key secondary endpoint.
Clinical trial identification
IFOM-CPT005/2019/PO004; EudraCT 2019-002074-32; EudraCT 2024-517704-10-00; NCT04259944.
Legal entity responsible for the study
IFOM ETS - the AIRC Institute of Molecular Oncology.
Funding
Guardant Health.
Disclosure
S. Marsoni: Other, Institutional, Research Grant: MSD, AstraZeneca, Illumina. N. Tarazona Llavero: Financial Interests, Personal, Advisory Board: Merck, Guardant Health, Grifols; Financial Interests, Personal, Invited Speaker: MERCK, Pfizer, Servier, Amgen; Financial Interests, Institutional, Funding: Natera Inc, Guardant Health; Non-Financial Interests, Member: SEOM Committee. C. Montagut Viladot: Financial Interests, Personal, Advisory Board: Merck Serono, Roche, Takeda; Financial Interests, Personal, Invited Speaker: Merck Serono, Pierre Fabre, Guardant Health, Amgen, Pfizer, MSD; Financial Interests, Institutional, Invited Speaker: MSD; Financial Interests, Institutional, Royalties: Biocartis; Financial Interests, Institutional, Coordinating PI: Merck-Serono; Non-Financial Interests, Member: TTD (Grupo Tratamiento Tumores Digestivos). A. Sartore Bianchi: Financial Interests, Personal, Invited Speaker: Amgen, Bayer, Pierre Fabre; Financial Interests, Personal, Advisory Board: Servier, Takeda. M.G. Zampino: Financial Interests, Personal, Advisory Board, Advisory Board for discussing the role of dostarlimab in rectal cancer: GSK. F.J. Ros Montana: Financial Interests, Institutional, Invited Speaker: Sanofi; Financial Interests, Personal, Invited Speaker: Pfizer, BMS, Takeda, Johnson and Johnson, Merck, Amgen; Other, Travel and accommodation expenses and Speaker: Amgen, Merck; Other, Travel and accommodation expenses: Pierre Fabre, Sanofi. C. Santos Vivas: Financial Interests, Personal, Advisory Board: Amgen; Financial Interests, Personal, Invited Speaker: Amgen, Takeda Pharmaceuticals; Other, Travel grant: Merck, MSD, Amgen, Takeda Pharmaceuticals. M. Mandalà: Financial Interests, Personal, Advisory Board: MSD, Novartis, Sanofi, BMS, Pierre Fabre; Financial Interests, Personal, Invited Speaker: Sun Pharma. S. Tamberi: Other, Personal, Speaker, Consultant, Advisor: AstraZeneca, Roche, Incute, Servier, Takeda. M.S. Sciallero: Financial Interests, Personal, Invited Speaker: MSD, Gentili; Financial Interests, Personal, Other, Consultancy on Lynch Syndrome: GSK; Other, Registration/Travel/accommodation expenses for congress: Novartis. S. Muñoz: Financial Interests, Institutional, Full or part-time Employment: Vall Hebron Institut d'Oncologia (VHIO); Financial Interests, Institutional, Other, Head of Academic CRO managing clinical trials: AstraZeneca Spain; Financial Interests, Institutional, Other, Head of academic CRO managing clinical trials: Roche, Bristol Myers Squibb, The Lymphoma Academic Research Organization, Leukos; Financial Interests, Institutional, Other, Head of CRO managing clinical trials: Pfizer, Pierre Fabre, Merck; Non-Financial Interests, Institutional, Product Samples, Head of academic CRO managing clinical trials: Merck, Leukos, Pierre Fabre, Pfizer, Roche, AstraZeneca, Bristol Myers Squibb, The Lymphoma Academic Research Organization. P. Luraghi: Financial Interests, Personal, Full or part-time Employment: AstraZeneca. E. Rulli: Financial Interests, Institutional, Research Grant, Roche supported an investigator initiated trial ,coordinated by our group: Roche; Financial Interests, Institutional, Research Grant, AstraZeneca supported investigator initiated trials, coordinated by our group: AstraZeneca. V. Torri: Financial Interests, Institutional, Funding, Roche supported an investigator initiated trial, coordinated by our department: Roche; Financial Interests, Institutional, Research Grant, AstraZeneca supported with unrestricted grant a study coordinated by our department: AstraZeneca. E. Elez Fernandez: Financial Interests, Personal, Advisory Board: Agenus Inc., Amgen, Bayer, Boehringer Ingelheim, Cure Teq AG, Hoffman La - Roche, Janssen, MSD, Merck Serono, Pierre Fabre, RIN Institute Inc., Repare Therapeutics Inc., Sanofi, Servier, Takeda, Johnson & Johnson, Rottapharm Biotech, Nordic Group BV; Financial Interests, Personal, Invited Speaker: BMS, Lilly, Medscape, Novartis, Organon, Pfizer; Financial Interests, Personal, Other, Steering Committee: GSK; Financial Interests, Personal, Other, Educational training: Seagen International GmbH; Financial Interests, Institutional, Funding: AbbVie Deutschland Gmbh & Co KG, Amgen Inc., Array Biopharma Inc, AstraZeneca Pharmaceuticals LP, Bayer Pharma AG, BeiGene, Bioncotech Therapeutics, S.L., Biontech Rna Pharmacuticals GMBH, BioNTech Small Molecules GMBH, Boehringer Ingelheim, Boehringer Ingelheim de España S.A., Bristol Myers Squibb International Corporation, Celgene International SARL, Daiichi Sankyo, Inc, Debiopharm International SA, Genentech Inc, Gercor, HalioDX SAS, Hoffmann-La Roche Ltd, Hutchinson Medipharma Limited, Hutchison MediPharma International, Iovance Biotherapeutics, Inc., Janssen Research & Development, Janssen-Cilag SA, MedImmune, Menarini, Menarini Ricerche SPA, Merck Health KGAA, Merck Sharp & Dohme de España SA, Merus NV, Mirati, Nouscom SRL., Novartis Farmacéutica SA, Pfizer, PharmaMar SA, Pledpharma AB, Redx Pharma PLC, Sanofi Aventis Recherche & Développement, Scandion Oncology, Seattle Genetics Inc., Servier, Sotio A.S., Taiho Pharma USA Inc, Wntresearch AB, Exelixis Inc., GSK, SA, Agenus, Nu Cana plc, Enterome BioScience SA; Non-Financial Interests, Leadership Role, Member of the Scientific Program Committee and Developmental Therapeutics-Immunotherapy Track Leader, 2023-2024 term: American Society for Clinical Oncology (ASCO); Non-Financial Interests, Other, Speaker of the ESMO Academy: European Society for Medical Oncology (ESMO); Non-Financial Interests, Other, Member of the Scientific Committee 2024: European Society for Medical Oncology (ESMO); Non-Financial Interests, Other, Coordinator of the SEOM +MIR Section of Residents and Young Assistants: Sociedad Española de Oncología Médica (SEOM); Non-Financial Interests, Other, Member of the Scientific Committee SEOM Annual Meeting, 2024-2025 term: Sociedad Española de Oncología Médica (SEOM); Non-Financial Interests, Member: American Society of Clinical Oncology (ASCO), American Association for Cancer Research (AACR), Sociedad Española de Oncología Médica (SEOM), Asociación Española de Investigación sobre el Cáncer (ASEICA), European Organization for Research and Treatment of Cancer (EORTC), Spanish Cooperative Gastrointestinal Tumors Group (TTD); Other, Travel, accommodations, expenses.: Agenus Inc., Amgen, BMS, Bayer, Boehringer Ingelheim, Cure Teq AG, GSK, Hoffman La-Roche, Janssen, Lilly, MSD, Medscape, Merck Serono, Novartis, Organon, Pfizer, Pierre Fabre, RIN Institute Inc., Repare Therapeutics Inc., Sanofi, Seagen, Servier, Takeda. A. Bardelli: Financial Interests, Personal, Other, Receipt of honoraria or consultation fees: Guardant Health; Financial Interests, Personal, Advisory Board, Scientific Advisory Board: Neophore; Financial Interests, Personal, Stocks/Shares, Stock shareholder: Neophore, Kither; Financial Interests, Institutional, Research Grant, Receipt of grants/research supports: Neophore, AstraZeneca, Boehringer Ingelheim. S. Siena: Financial Interests, Personal, Advisory Board, Advisory Board Member: Agenus, AstraZeneca, BMS, Checkmab, Daiichi Sankyo, GSK, Novartis, Seagen, T-One-Therapeutics, Merck, MSD. S. Lonardi: Financial Interests, Personal, Advisory Board: Amgen, Merck Serono, Lilly, Servier, AstraZeneca, MSD, Incyte, Daiichi Sankyo, Bristol Myers Squibb, Astellas, GSK, Takeda, Bayer, Rottapharm, BeiGene, Nimbus Therapeutics, Helion; Financial Interests, Personal, Invited Speaker: Pierre Fabre, GSK, Roche, Servier, Amgen, Bristol Myers Squibb, Incyte, Lilly, Merck Serono, MSD, AstraZeneca; Financial Interests, Institutional, Coordinating PI: Amgen, Merck Serono, Bayer, Roche, Lilly, AstraZeneca, Bristol Myers Squibb; Non-Financial Interests, Member of Board of Directors, Italian No-Profit Oncology Research Foundation supporting academic Clinical trials: GONO Foundation. All other authors have declared no conflicts of interest.
LBA8 - IMvigor011: A phase III trial of circulating tumour (ct)DNA-guided adjuvant atezolizumab vs placebo in muscle-invasive bladder cancer
- Thomas B. Powles (London, United Kingdom)
- Thomas B. Powles (London, United Kingdom)
- Ariel Kann (Sao Paulo, Brazil)
- Daniel E. Castellano Gauna (Madrid, Spain)
- Marine Gross Goupil (Bordeaux, France)
- Hiroyuki Nishiyama (Tsukuba, Japan)
- Sergio Bracarda (Terni, Italy)
- Jørgen Bjerggaard Jensen (Aarhus, Denmark)
- Shusuan Jiang (Changsha, China)
- Ja Hyeon Ku (Seoul, Republic of Korea)
- Marco Maruzzo (Padova, Italy)
- Dingwei Ye (Shanghai, China)
- Rafael Morales Barrera (Barcelona, Spain)
- Oscar Reig Torras (Barcelona, Spain)
- Andrea Necchi (Milan, Italy)
- Wei Zou (South San Francisco, United States of America)
- Zoe June F. Assaf (South San Francisco, United States of America)
- Jacqueline Vuky (Portland, United States of America)
- Elizabeth Steinberg (San Francisco, United States of America)
- Joaquim Bellmunt (Boston, United States of America, MA)
- Juergen Gschwend (Munich, Germany)
Background
Serial monitoring of ctDNA-based molecular residual disease after radical cystectomy in patients (pts) with high-risk muscle-invasive bladder cancer shows promise in differentiating pts who need adjuvant therapy from those who can safely avoid treatment. We report the primary analysis of the global, randomised, double-blind, phase 3 IMvigor011 trial (NCT04660344) studying ctDNA-guided atezolizumab (atezo) vs placebo in this setting.
Methods
Pts with muscle-invasive bladder cancer and no radiographic evidence of disease enrolled in surveillance within 6–24 weeks of cystectomy and underwent serial ctDNA monitoring for up to 1 year after surgery. Eligible pts who tested ctDNA+ were randomised 2:1 to atezo 1680 mg or placebo every 4 weeks for 12 cycles or up to 1 year. The primary endpoint was investigator-assessed disease-free survival (DFS). Overall survival (OS) was a secondary endpoint with alpha control. Pts who persistently tested ctDNA− received no treatment.
Results
Overall, 761 pts were enrolled in surveillance. 250 eligible pts who tested ctDNA+ were randomised (atezo, n=167; placebo, n=83). At a median follow-up of 16.1 months, pts in the atezo arm had statistically significant improvements in DFS (HR, 0.64; 95% CI: 0.47, 0.87;
Randomised ctDNA+
| Atezo (n=167) | Placebo (n=83) | |
| Events, n | 112 | 66 |
| Median (95% CI), months | 9.9 (7.2, 12.7) | 4.8 (4.1, 8.3) |
| Stratified HR (95% CI)b | 0.64 (0.47, 0.87); |
|
| 12-month rate, % | 44.7 | 29.6 |
| Events, n | 60 | 36 |
| Median (95% CI), months | 32.8 (27.7, NE) | 21.1 (14.7, NE) |
| Stratified HR (95% CI)b | 0.59 (0.39, 0.90); |
|
| 12-month rate, % | 85.1 | 70.0 |
aPer investigator. bStratification factors: nodal status, tumour stage, programmed death-ligand 1 status and time from radical cystectomy to first ctDNA+ sample. cPrespecified interim analysis. CI, confidence interval; HR, hazard ratio; NE, not evaluable.
Conclusions
ctDNA-guided adjuvant atezo showed statistically significant and clinically meaningful DFS and OS improvements vs placebo. The atezo safety profile was tolerable, with no new findings. Pts who persistently tested ctDNA− had low risk of recurrence.
Clinical trial identification
NCT04660344.
Editorial acknowledgement
Medical writing assistance for this abstract was provided by Bena Lim, PhD, CMPP, of Nucleus Global, an Inizio Company, and funded by F. Hoffmann-La Roche Ltd.
Legal entity responsible for the study
F. Hoffmann-La Roche Ltd.
Funding
F. Hoffmann-La Roche Ltd.
Disclosure
T.B. Powles: Financial Interests, Personal, Advisory Board: AstraZeneca, Bristol Myers-Squibb, Exelixis, Incyte, Ipsen, Merck, Novartis, Pfizer, Seattle Genetics, Merck Serono, Astellas, Johnson & Johnson, Eisai, Roche, MSD; Financial Interests, Personal, Other, Travel/Accommodation/Expenses: Roche, Pfizer, MSD, AstraZeneca, Ipsen; Financial Interests, Personal, Other, Sponsorship for Uromigos Podcast: Mashup Ltd; Financial Interests, Institutional, Other, honoraria: Gilead; Financial Interests, Institutional, Research Grant: AstraZeneca, Roche, Bristol Myers-Squibb, Exelixis, Ipsen, Merck, MSD, Seattle Genetics, Novartis, Pfizer, Merck Serono, Astellas, Johnson & Johnson, Eisai; Financial Interests, Institutional, Other, Honoraria: Gilead. A. Kann: Non-Financial Interests, Personal, Other, Third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd. D.E. Castellano Gauna: Non-Financial Interests, Personal, Other, Third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd. M. Gross Goupil: Financial Interests, Personal, Speaker, Consultant, Advisor: BMS, Pfizer, Astellas, MSD, Gilead; Financial Interests, Personal, Research Funding: BMS, Pfizer, Astellas, MSD, Gilead, Roche; Financial Interests, Personal, Other, Honoraria: BMS, Pfizer, Astellas, MSD , Gilead; Non-Financial Interests, Personal, Other, Third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd. H. Nishiyama: Financial Interests, Personal, Research Funding: Chugai Pharma Astellas; Financial Interests, Personal, Other, Honoraria: Merck Sharp & Dohme (MSD), Ono Pharmaceutical Janssen; Financial Interests, Personal, Speaker’s Bureau: Astellas , Merck Sharp & Dohme (MSD), Merck Biopharma Co.Ltd, Nippon Kayaku Co.Ltd. , Ono Pharmaceutical , Bristol Myers Squibb (BMS) , Janssen, Pfizer; Non-Financial Interests, Personal, Other, Third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd. S. Bracarda: Financial Interests, Personal, Other: Bayer, Astellas, Johnson&Johnson, MSD, BMS, Roche-Genentech, Ipsen, Pfizer, Novartis, AstraZeneca, Merck, Gilead, Indicon, Indicon; Non-Financial Interests, Personal, Other, Third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd. J.B. Jensen: Financial Interests, Personal, Research Funding: Medac, Photocure ASA, Roche, Ferring, Olympus, Intuitive Surgery, Astellas, Cepheid, Nucleix, Urotech, Pfizer, AstraZeneca, MeqNordic, Laborie, OneMed, AMBU, Cystotech; Financial Interests, Personal, Other, Honoraria: MSD; Financial Interests, Personal, Advisory Board: Ferring, Roche, Cepheid, Urotech, Olympus, AMBU, Janssen, Cystotech. S. Jiang, J.H. Ku: Non-Financial Interests, Personal, Other, Third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd. M. Maruzzo: Financial Interests, Personal, Other, Consultancy: BMS, MSD, Merck Serono, Astellas, Ipsen, Eisai, Recordati, Jonhson & Jonhson, AstraZeneca; Non-Financial Interests, Personal, Other, Third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd. D. Ye: Non-Financial Interests, Personal, Other, Third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd. R. Morales Barrera: Financial Interests, Personal, Other, Honoraria: Merck Group, Lilly, AstraZeneca, Merck, Sharp & Dohme, Astellas; Financial Interests, Personal, Speaker’s Bureau: Merck Group, Lilly, AstraZeneca, Merck, Sharp & Dohme, Astellas; Financial Interests, Personal, Other, Travel and accommodations expenses: Roche, Sanofi Aventis, Janssen, Merck Sharp & Dohme, Bayer, BMS, Merck Group; Non-Financial Interests, Personal, Other, third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd. O. Reig Torras: Financial Interests, Personal, Stocks/Shares: BMS, MSD, Pfizer; Financial Interests, Personal, Other, Honoraria: BMS, Ipsen, Pfizer, Astellas , Pharma, Merck; Financial Interests, Personal, Speaker’s Bureau: Pfizer, Ipsen, Sanofi, BMS, Janssen, Bayer; Non-Financial Interests, Personal, Advisory Board, third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd. A. Necchi: Financial Interests, Institutional, Research Grant: Merck, AstraZeneca, Ipsen, BMS, Gilead; Financial Interests, Personal, Steering Committee Member: Janssen, Astellas, AstraZeneca, Merck, Gilead, BMS, Bicycle Therapeutics, Daiichi Sankyo; Financial Interests, Coordinating PI: Incyte, Genenta Sciences; Financial Interests, Personal, Coordinating PI: Catalym; Financial Interests, Personal, Funding: Samsung Bioepis; Non-Financial Interests, Leadership Role: Global society of Rare Genitourinary Tumors (GSRGT). W. Zou: Financial Interests, Personal, Stocks or ownership: F. Hoffmann-La Roche Ltd; Non-Financial Interests, Personal, Other, third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd; Financial Interests, Personal, Full or part-time Employment: Genentech, Inc. Z.J.F. Assaf: Non-Financial Interests, Personal, Other, third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd; Financial Interests, Personal, Stocks/Shares: F. Hoffmann-La Roche Ltd; Financial Interests, Personal, Full or part-time Employment: Genentech, Inc. J. Vuky: Financial Interests, Personal, Stocks/Shares: F. Hoffmann-La Roche Ltd; Non-Financial Interests, Personal, Other, third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd; Financial Interests, Personal, Full or part-time Employment: Genentech Inc. E. Steinberg: Financial Interests, Personal, Stocks/Shares: F. Hoffmann-La Roche Ltd; Non-Financial Interests, Personal, Other, third-party medical writing assistance, furnished by Bena Lim, PhD, of Nucleus Global, an Inizio Company, from F. Hoffmann-La Roche Ltd: F. Hoffmann-La Roche Ltd; Financial Interests, Personal, Full or part-time Employment: Genentech Inc. J. Bellmunt: Financial Interests, Personal, Advisory Board, Joined the Global adboard this year: Pfizer; Financial Interests, Personal, Advisory Board, Regular GU adboard for bladder cancer: Astra-Zeneca; Financial Interests, Personal, Invited Speaker, Lectures in the setting of National meetings: Merck; Financial Interests, Personal, Advisory Board, Bladder adboard: Merck; Financial Interests, Personal, Advisory Board, For the adjuvant study CM 247: BMS; Financial Interests, Personal, Invited Speaker, For ESMO Asia Symp 2020: MSD; Financial Interests, Personal, Stocks/Shares, Holdings: Bicycle; Financial Interests, Personal, Royalties, Role as Section Editor for Bladder: UpToDate; Financial Interests, Institutional, Coordinating PI, Pi of INDUCOMAIN Study (Avelumab first line in unfit patients) Though APRO Association: MSD; Financial Interests, Institutional, Coordinating PI, Pi of Prostate Study (Avelumab + Carboplatin) Though APRO Association.: Pfizer; Non-Financial Interests, Other, Steering committee member of IMvigor 011: Genentech; Non-Financial Interests, Member: ASCO. J. Gschwend: Financial Interests, Personal, Advisory Board: BMS, Roche, Janssen, Merck, Pfizer, Astellas, Novartis, MSD.
Gerelateerde artikelen
- ctDNA in het bloed van patiënten met endeldarmkanker en dikke darmkanker wordt meer en meer toegepast. Hier een overzicht en enkele lopende studies met ctDNA
- ctDNA: multimodale ctDNA bloedtest die genomica en epigenetica integreert in combinatie met proteomics constateert 84 tot 96 procent juiste stadium van darmkanker na verdenking in screening en bevolkingsonderzoek
- ctDNA - circulerend tumor DNA is uitstekende biomarker voor wel of geen toekomstig recidief na operatie en of chemotherapie bij endeldarmkanker - rectumkanker blijkt uit meta-analyse
- Regulier - Darmkankers: recente ontwikkelingen en belangrijke studies - artikelen over behandelen en omgaan met vormen van darmkanker binnen de reguliere oncologie: een overzicht



Plaats een reactie ...
Reageer op "ctDNA - circulerend tumor-DNA via bloed verkregen toont in de klinische praktijk geen meerwaarde t.o.v. chemotherapie maar afbouwen van chemotherapie na negatieve ctDNA test speelt daarin cruciale rol"