Helpt u ons aan 500 donateurs?
20 februari 2020: Ook een nieuwe gerandomiserde placebo gecontroleerde studie bevestigt dat wanneer mensen waarvan in hun directe familie (ouders, kinderen) bekend is dat deze besmet zijn geweest met de Helicobacter pylori bacterie en daardoor maagkanker hadden gekregen en nu bij hun zelf actief de H. pylori bacterie werd bestreden en gedood dan hebben zij meer dan de helft minder risico (1,2 versus 2,7 procent) op het ontstaan van maagkanker binnen 9 jaar. Wat logisch is want de Heliobacter Pylori bacterie is voor een groot gedeelte verantwoordelijk voor het ontstaan van maagkanker.
Het gaat om deze studie: Family History of Gastric Cancer and Helicobacter pylori Treatment
In een gerandomiseerde, gecontroleerde studie uit Korea werden 1676 patiënten met Helicobacter pylori-infectie en een eerstegraads familielid met maagkanker gerandomiseerd naar de effecten van een driedubbele bestrijdingsbehandeling of placebo. Surveillance-endoscopieën werden om de 2 jaar uitgevoerd. H. pylori werd uitgeroeid bij 70,1% van de behandelingsgroep en bij 7,1% van de placebogroep.
Tijdens een mediane follow-up periode van 9,2 jaar ontwikkelde maagkanker zich bij 1,2% van de behandelingsgroep versus 2,7% van de placebo-groep (HR, 0,45). Maagkanker ontwikkelde zich bij 2,9% van de patiënten met een aanhoudende H. pylori-infectie en 0,8% bij patiënten met succesvolle uitroeiing van de H. pylori bacterie (HR, 0,27).
Lees ook verderop in dit artikel en in gerelateerde artikelen hoe je met voeding en voedingsstoffen ook heel goed de H. pylori bacterie kunt elimineren, zie ook o.a. dit studierapport: Medicinal plant activity on Helicobacter pylori related diseases).
31 maart 2018: Bron: N Engl J Med 2018; 378:1085-1095
Wanneer mensen waarbij een endoscopische operatie van vroege maagkanker is uitgevoerd of sprake is van een hooggradig adenoom daarna een behandeling krijgen met antibiotica gericht op het elimineren van de H. pylori bacterie dan geeft dat betere resultaten in vergelijking met een placebo.
Lees ook verderop in dit artikel en in gerelateerde artikelen hoe je met voeding en voedingsstoffen ook heel goed de H. pylori bacterie kunt elimineren, zie ook o.a. dit studierapport: Medicinal plant activity on Helicobacter pylori related diseases).
Citaat uit die studie: Many medicinal plant products possess anti-Helicobacter pylori (H. pylori) activity as well as an anti-H. pylori induced gastric inflammatory effect. Those plant products have showed great potential as pharmaceutical candidates for H. pylori eradication and H. pylori induced related gastric disease prevention.
Onder foto gaat tekst door.
 H. Pylori en maagkanker
H. Pylori en maagkanker
Uit een recente placebo gecontroleerde gerandomiserde studie kwam naar voren dat
1. Er zich minder ontwikkelende maagkanker werd ontdekt bij een endoscopie een jaar later.
2. 3 jaar later zagen de onderzoekers een verbetering ten opzichte van de uitgangswaarden van de ernst van de zogeheten glandulaire atrofie bij de follow-up meting na 3 jaar.
Uit het abstract van de studie:
-
This study was a prospective, double-blind, placebo-controlled, randomized trial that assigned 470 patients who had undergone endoscopic resection of early gastric cancer or high-grade adenoma to receive either H. pylori eradication therapy with antibiotics or placebo. A total of 396 patients were included in the modified intention-to-treat analysis population (194 in the treatment group and 202 in the placebo group). During a median follow-up of 5.9 years, metachronous gastric cancer developed in 14 patients (7.2%) in the treatment group and in 27 patients (13.4%) in the placebo group (treatment group HR, 0.50; P = .03). Among the 327 patients in the subgroup that underwent histologic analysis, improvement from baseline in the atrophy grade at the gastric corpus lesser curvature was observed in 48.4% of the patients in the treatment group and in 15.0% of those in the placebo group (P < .001). There were no serious adverse events; mild adverse events were more common in the treatment group (42.0% vs 10.2%; P < .001).
-
Patients with early gastric cancer who received H. pylori treatment had lower rates of metachronous gastric cancer and more improvement from baseline in the grade of gastric corpus atrophy than patients who received placebo.
Het volledige studierapport: Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer is tegen betaling in te zien.
30 mei 2017: Bron de Volkskrant en WJG - World Journal of Gastroenterology
In de Volkskrant wetenschappelijke rubriek VOEDING werd afgelopen week deze vraag behandeld: Kan voeding een maagzweer laten verdwijnen?
N.a.v. daarvan hier een reviewstudie uit 2014 over ditzelfde onderwerp: Exploring alternative treatments for Helicobacter pylori infection
en uit 2016 een reviewstudie: Nutrition and Helicobacter pylori: Host Diet and Nutritional Immunity Influence Bacterial Virulence and Disease Outcome en een nieuwe publicatie over de rol van probiotica in de bestrijding van de Helico bacter Pylori, de veroorzaker van maagzweren en meestal ook de bron van maagkanker: Role of Probiotics in Managing of Helicobacter Pylori Infection: A Review
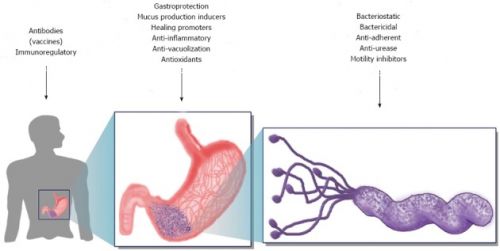
Bron: WJG 2014: Mechanism used in the alternative strategies described here for the treatment of Helicobacter pylori. Three levels are considered as targets for the different alternative treatments. The first involves the host, where vaccines and immune response modulators could act. The second is the stomach, where many mechanisms could have different types of action to restore homeostasis (i.e., gastroprotection, anti-inflammatory). Finally, Helicobacter pylori is the central target; in this case, alternative treatments are intended to eradicate or prevent the infection, acting upon growth or colonisation factors. In the case of growth inhibitors, many bacterial targets could be used as key enzymes and pathways.
Een paar citaten uit het artikel van de Volkskrant:
Kan voeding een maagzweer laten verdwijnen?
.........Een maagzweer wordt vaak veroorzaakt door de bacterie Helicobacter pylori, die zich nestelt in de beschermende slijmlaag van de maagwand. De bacterie veroorzaakt een chronische ontsteking van het maagslijmvlies. Daar merk je meestal niets van, verduidelijkt Ernst Kuipers, hoogleraar maag- darm- leverziektes in het Erasmus MC. Maar soms tast die ontsteking het slijmvlies en de slijmlaag aan waarna het maagzuur op die plek kan inwerken en een wond veroorzaakt. De zenuwen eronder komen bloot te liggen en als het maagzuur ermee in contact komt, ontstaat heftige pijn. De eerste maagzweerdiëten dateren van een eeuw geleden. Beroemd was het Sippy-dieet, vernoemd naar de Amerikaanse arts Bertram Sippy, die zijn patiënten dagenlang alleen melk gaf..........Het melkdruppel-dieet bleek niet te werken.
Doeltreffender is het vermijden van bepaalde dranken. Vooral koffie en alcohol zijn boosdoeners omdat ze de aanmaak van maagzuur stimuleren............
Een dieet tegen maagzuur is niet meer zo nodig, zegt Kuipers: er zijn geneesmiddelen, maagzuurremmers, die de productie van zuur in de maag veel beter afremmen dan welk voedingsmiddel dan ook.
Veel interessanter vindt hij het om te kijken naar voeding die de oorzaak van de zweer aanpakt: 'Als je de groei van de bacterie kunt afremmen, helpt dat om de ontsteking tegen te gaan.'.............
Het sterkste bewijs ligt er voor broccolispruiten (de kiemen van de broccoliplant), zwarte bessenolie, cranberrysap, kaneel en bepaalde probiotica. Die voedingsmiddelen pakken de Helicobacter-bacterie op uiteenlopende manieren aan: ze remmen de groei of het venijn van de bacterie, beperken de mogelijkheid om zich in de maagwand te nestelen of voorkomen dat de bacterie een beschermde jas krijgt waardoor het lastig wordt om in de zure maag te overleven.........
Kuipers wijst erop dat er geen antibioticum bestaat dat alleen de Helicobacter doodt. 'Bestrijden we één soort, dan raken we ook altijd andere soorten.'
Reden genoeg om een dieet een kans te geven. Maar voordat maagpatiënten hun medicijnen inruilen voor veenbessensap of kiemgroenten: eerst moet veel meer onderzoek worden gedaan..........>>>>>>Lees het hele artikel: Kan voeding een maagzweer laten verdwijnen?
Uit de studie: Nutrition and Helicobacter pylori: Host Diet and Nutritional Immunity Influence Bacterial Virulence and Disease Outcome het volgende citaat:
4. Helicobacter pylori en Voeding
Naast genetische verschillen bij de individuele mens of stammen, komen milieufactoren, zoals het dieet van de individuele mens, op als belangrijke componenten van de ecologie binnen de maagomgeving. Het is waarschijnlijk dat de maagomgeving sterk beïnvloed wordt door het voedingspatroon van de individuele mens. Epidemiologische studies hebben aangetoond dat voedingsgewoonten zoals hoge inname van groene thee, fruit of groenten beschermend zijn tegen maagkankerrisico [zie in referenties 37–39]. Omgekeerd blijkt uit case-controlled en cohort studies dat de hoge inname van rood vlees en / of verwerkt vlees (die veel overgangsmetalen bevatten) en conserven (gepekeld, gedroogd, gerookt of gezouten) die vaak zout bevatten, geassocieerd zijn met een verhoogd risico op 'noncardia' maagkanker [zie referenties 40, 41]. Bovendien heeft de komst van de koelkast en vrieskast radicaal de manier waarop voedsel voor opslag wordt voorbereid veranderd. Case-controlled populatiestudies hebben aangetoond dat toegang tot koeling beschermend is tegen maagkanker [zie referentie 42]. Dit is te wijten aan het feit dat koeling leidt tot langdurige toegang tot vers voedsel zoals fruit en groenten, die anders niet beschikbaar zouden zijn. Er wordt verondersteld dat carotenoïden, foliumzuur, vitamine C en fytochemicaliën van groenten en fruit een beschermende rol hebben tegen carcinogenese ( vorming van kankercellen). Omgekeerd kunnen zout en de beschikbaarheid van sommige overgangsmetalen de H. pylori activiteit veranderen en vorming van kwaadaardige tumorcellen (carcinogenese) versnellen [zie referenties: 43, 44]. De bijdrage van deze individuele micronutriënten aan H. pylori-afhankelijke ziekten wordt hieronder (zie volledige studierapprot) nader onderzocht.
Oorspronkelijke tekst van de tekst hierboven zoals ik die heb vertaald:
4. H. pylori and Nutrition
In addition to host or strain genetic differences, environmental factors, such as host diet, are emerging as important components of the ecology within the gastric environment. It is likely that the gastric environment is highly influenced by host nutrient intake. Epidemiological studies have revealed that dietary habits such as high intake of green tea, fruits, or vegetables are protective against gastric cancer risk [37–39]. Conversely, case-controlled and cohort studies reveal that high intake of red meat and/or processed meat (which are high in transition metals) and preserved foods (pickled, dried, smoked, or salted) which are often high in salt is associated with increased risk of noncardia gastric cancer [40, 41]. Furthermore, the advent of refrigeration has radically changed the manner in which food is prepared for storage. Case-controlled population studies have demonstrated that access to refrigeration is protective against gastric cancer [42]. This is attributed to the fact that refrigeration leads to prolonged access to fresh foods such as fruits and vegetables, which would otherwise be unavailable. It is hypothesized that carotenoids, folate, vitamin C, and phytochemicals from fruits and vegetables have a protective role against carcinogenesis. Conversely, salt and the availability of some transition metals can alter H. pylori virulence and accelerate carcinogenesis [43, 44]. The contribution of these individual micronutrients to H. pylori-dependent diseases will be reviewed in detail below.
Het blijkt dus dat voeding een grote rol kan spelen in het voorkomen van de heliobacter Pyl;ori bacterie, maar niet duidelijjk is precies welke voedingstoffen precies het beste werken. Wat veel onderzocht is zijn de verschiullende melkzuurbacteriën (probiotica).
In deze studie: The Effect of Probiotics on Gut Microbiota during the Helicobacter pylori Eradication: Randomized Controlled Trial. concluderen de onderzoekers:
Probiotische supplementen kunnen de antibiotica-geïnduceerde verandering en onevenwichtigheid van de darmflora - darmmicrobiota samenstelling verminderen. Dit effect kan de groei van antibiotica resistente bacteriën in de darm beperken en de eliminering van de H. pylori bacterie versnellen. (oorspronkelijke tekst: Probiotic supplementation can reduce the antibiotic-induced alteration and imbalance of the gut microbiota composition. This effect may restrict the growth of antibiotic-resistant bacteria in the gut and improve the H. pylori eradication success rate.
Maar leest u de genoemde studies mocht u mer willen weten.
Hieronder enkele abstracten met referentielijsten:
The intersection of host genetics, immune response, bacterial virulence expression, diet, micronutrient availability, and microbiome structure and composition undoubtedly influence the disease outcomes associated with chronic Helicobacter pylori infection.
Nutrition and Helicobacter pylori: Host Diet and Nutritional Immunity Influence Bacterial Virulence and Disease Outcome
Abstract
Helicobacter pylori colonizes the stomachs of greater than 50% of the world's human population making it arguably one of the most successful bacterial pathogens. Chronic H. pylori colonization results in gastritis in nearly all patients; however in a subset of people, persistent infection with H. pylori is associated with an increased risk for more severe disease outcomes including B-cell lymphoma of mucosal-associated lymphoid tissue (MALT lymphoma) and invasive adenocarcinoma. Research aimed at elucidating determinants that mediate disease progression has revealed genetic differences in both humans and H. pylori which increase the risk for developing gastric cancer. Furthermore, host diet and nutrition status have been shown to influence H. pylori-associated disease outcomes. In this review we will discuss how H. pylori is able to create a replicative niche within the hostile host environment by subverting and modifying the host-generated immune response as well as successfully competing for limited nutrients such as transition metals by deploying an arsenal of metal acquisition proteins and virulence factors. Lastly, we will discuss how micronutrient availability or alterations in the gastric microbiome may exacerbate negative disease outcomes associated with H. pylori colonization.
References
1.
Moodley Y., Linz B., Bond R. P., et al. Age of the association between Helicobacter pylori and man. PLoS Pathogens. 2012;8(5) doi: 10.1371/journal.ppat.1002693.e1002693 [PMC free article] [PubMed] [Cross Ref]2.
Kodaman N., Pazos A., Schneider B. G., et al. Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(4):1455–1460. doi: 10.1073/pnas.1318093111. [PMC free article] [PubMed] [Cross Ref]3.
Kodaman N., Sobota R. S., Mera R., Schneider B. G., Williams S. M. Disrupted human-pathogen co-evolution: a model for disease. Frontiers in Genetics. 2014;5, article 290 doi: 10.3389/fgene.2014.00290. [PMC free article] [PubMed] [Cross Ref]4.
Parkin D. M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [PubMed] [Cross Ref]5.
Polk D. B., Peek R. M., Jr. Helicobacter pylori: gastric cancer and beyond. Nature Reviews Cancer. 2010;10(6):403–414. doi: 10.1038/nrc2857. [PMC free article] [PubMed] [Cross Ref]6.
Correa P., Piazuelo M. B. The gastric precancerous cascade. Journal of Digestive Diseases. 2012;13(1):2–9. doi: 10.1111/j.1751-2980.2011.00550.x. [PMC free article] [PubMed] [Cross Ref]7.
Schneider B. G., Mera R., Piazuelo M. B., et al. DNA methylation predicts progression of human gastric lesions. Cancer Epidemiology Biomarkers & Prevention. 2015;24(10):1607–1613. [PMC free article] [PubMed]8.
Wei J., Noto J. M., Zaika E., et al. Bacterial CagA protein induces degradation of p53 protein in a p14ARF-dependent manner. Gut. 2015;64(7):1040–1048. doi: 10.1136/gutjnl-2014-307295. [PMC free article] [PubMed] [Cross Ref]9.
Wei J., Nagy T. A., Vilgelm A., et al. Regulation of p53 tumor suppressor by helicobacter pylori in gastric epithelial cells. Gastroenterology. 2010;139(4):1333–1343. doi: 10.1053/j.gastro.2010.06.018. [PMC free article] [PubMed] [Cross Ref]10.
Hardbower D. M., Peek R. M., Jr., Wilson K. T. At the bench: Helicobacter pylori, dysregulated host responses, DNA damage, and gastric cancer. Journal of Leukocyte Biology. 2014;96(2):201–212. doi: 10.1189/jlb.4bt0214-099r. [PMC free article] [PubMed] [Cross Ref]11.
Gil R., Sabater-Muñoz B., Latorre A., Silva F. J., Moya A. Extreme genome reduction in Buchnera spp.: toward the minimal genome needed for symbiotic life. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(7):4454–4458. doi: 10.1073/pnas.062067299. [PMC free article] [PubMed] [Cross Ref]12.
Dong Q.-J., Wang L.-L., Tian Z.-B., Yu X.-J., Jia S.-J., Xuan S.-Y. Reduced genome size of Helicobacter pylori originating from East Asia. World Journal of Gastroenterology. 2014;20(19):5666–5671. doi: 10.3748/wjg.v20.i19.5666. [PMC free article] [PubMed] [Cross Ref]13.
Fadiel A., Eichenbaum K. D., El Semary N., Epperson B. Mycoplasma genomics: tailoring the genome for minimal life requirements through reductive evolution. Frontiers in Bioscience. 2007;12(6):2020–2028. doi: 10.2741/2207. [PubMed] [Cross Ref]14.
Ghoshal U. C., Tiwari S., Dhingra S., et al. Frequency of Helicobacter pylori and CagA antibody in patients with gastric neoplasms and controls: the Indian enigma. Digestive Diseases and Sciences. 2008;53(5):1215–1222. doi: 10.1007/s10620-008-0229-7. [PubMed] [Cross Ref]15.
Goh K.-L., Cheah P.-L., Md N., Quek K.-F., Parasakthi N. Ethnicity and H. pylori as risk factors for gastric cancer in Malaysia: a prospective case control study. The American Journal of Gastroenterology. 2007;102(1):40–45. doi: 10.1111/j.1572-0241.2006.00885.x. [PubMed] [Cross Ref]16.
Bravo L. E., Van Doorn L.-J., Realpe J. L., Correa P. Virulence-associated genotypes of Helicobacter pylori: do they explain the African enigma? American Journal of Gastroenterology. 2002;97(11):2839–2842. doi: 10.1111/j.1572-0241.2002.07031.x. [PubMed] [Cross Ref]17.
Con S. A., Valerín A. L., Takeuchi H., et al. Helicobacter pylori CagA status associated with gastric cancer incidence rate variability in Costa Rican regions. Journal of Gastroenterology. 2006;41(7):632–637. doi: 10.1007/s00535-006-1812-3. [PubMed] [Cross Ref]18.
Lin D., Koskella B. Friend and foe: factors influencing the movement of the bacterium Helicobacter pylori along the parasitism-mutualism continuum. Evolutionary Applications. 2015;8(1):9–22. doi: 10.1111/eva.12231. [PMC free article] [PubMed] [Cross Ref]19.
Engler D. B., Reuter S., Van Wijck Y., et al. Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(32):11810–11815. doi: 10.1073/pnas.1410579111. [PMC free article] [PubMed] [Cross Ref]20.
Correa P., Cuello C., Duque E., et al. Gastric cancer in Colombia. III. Natural history of precursor lesions. Journal of the National Cancer Institute. 1976;57(5):1027–1035. [PubMed]21.
Segal E. D., Lange C., Covacci A., Tompkins L. S., Falkow S. Induction of host signal transduction pathways by Helicobacter pylori. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(14):7595–7599. doi: 10.1073/pnas.94.14.7595. [PMC free article] [PubMed] [Cross Ref]22.
Sharma S. A., Tummuru M. K. R., Blaser M. J., Kerr L. D. Activation of IL-8 gene expression by Helicobacter pylori is regulated by transcription factor nuclear factor-κB in gastric epithelial cells. Journal of Immunology. 1998;160(5):2401–2407. [PubMed]23.
Tummuru M. K. R., Sharma S. A., Blaser M. J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Molecular Microbiology. 1995;18(5):867–876. doi: 10.1111/j.1365-2958.1995.18050867.x. [PubMed] [Cross Ref]24.
Hofman V., Ricci V., Galmiche A., et al. Effect of Helicobacter pylori on polymorphonuclear leukocyte migration across polarized T84 epithelial cell monolayers: role of vacuolating toxin VacA and cag pathogenicity island. Infection and Immunity. 2000;68(9):5225–5233. doi: 10.1128/iai.68.9.5225-5233.2000. [PMC free article] [PubMed] [Cross Ref]25.
Su B., Ceponis P. J. M., Sherman P. M. Cytoskeletal rearrangements in gastric epithelial cells in response to Helicobacter pylori infection. Journal of Medical Microbiology. 2003;52, part 10:861–867. doi: 10.1099/jmm.0.05229-0. [PubMed] [Cross Ref]26.
McClain M. S., Iwamoto H., Cao P., et al. Essential role of a GXXXG motif for membrane channel formation by Helicobacter pylori vacuolating toxin. The Journal of Biological Chemistry. 2003;278(14):12101–12108. doi: 10.1074/jbc.m212595200. [PubMed] [Cross Ref]27.
Cover T. L., Blanke S. R. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nature Reviews Microbiology. 2005;3(4):320–332. doi: 10.1038/nrmicro1095. [PubMed] [Cross Ref]28.
Cover T. L., Vaughn S. G., Cao P., Blaser M. J. Potentiation of Helicobacter pylori vacuolating toxin activity by nicotine and other weak bases. Journal of Infectious Diseases. 1992;166(5):1073–1078. doi: 10.1093/infdis/166.5.1073. [PubMed] [Cross Ref]29.
Merrell D. S., Thompson L. J., Kim C. C., et al. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infection and Immunity. 2003;71(11):6510–6525. doi: 10.1128/iai.71.11.6510-6525.2003. [PMC free article] [PubMed] [Cross Ref]30.
Moonens K., Gideonsson P., Subedi S., et al. Structural insights into polymorphic ABO glycan binding by Helicobacter pylori. Cell Host & Microbe. 2016;19(1):55–66. doi: 10.1016/j.chom.2015.12.004. [PMC free article] [PubMed] [Cross Ref]31.
Ishijima N., Suzuki M., Ashida H., et al. BabA-mediated adherence is a potentiator of the Helicobacter pylori type IV secretion system activity. The Journal of Biological Chemistry. 2011;286(28):25256–25264. doi: 10.1074/jbc.m111.233601. [PMC free article] [PubMed] [Cross Ref]32.
Mahdavi J., Sondén B., Hurtig M., et al. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297(5581):573–578. doi: 10.1126/science.1069076. [PMC free article] [PubMed] [Cross Ref]33.
Peck B., Ortkamp M., Diehl K. D., Hundt E., Knapp B. Conservation, localization and expression of HopZ, a protein involved in adhesion of Helicobacter pylori. Nucleic Acids Research. 1999;27(16):3325–3333. doi: 10.1093/nar/27.16.3325. [PMC free article] [PubMed] [Cross Ref]34.
Giannakis M., Bäckhed H., Chen S. L., et al. Response of gastric epithelial progenitors to Helicobacter pylori isolates obtained from Swedish patients with chronic atrophic gastritis. The Journal of Biological Chemistry. 2009;284(44):30383–30394. doi: 10.1074/jbc.m109.052738. [PMC free article] [PubMed] [Cross Ref]35.
Franco A. T., Johnston E., Krishna U., et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Research. 2008;68(2):379–387. doi: 10.1158/0008-5472.can-07-0824. [PMC free article] [PubMed] [Cross Ref]36.
Aspholm M., Olfat F. O., Nordén J., et al. SabA is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans. PLoS Pathogens. 2006;2(10, article e110) doi: 10.1371/journal.ppat.0020110. [PMC free article] [PubMed] [Cross Ref]37.
Setiawan V. W., Zhang Z.-F., Yu G.-P., et al. Protective effect of green tea on the risks of chronic gastritis and stomach cancer. International Journal of Cancer. 2001;92(4):600–604. doi: 10.1002/ijc.1231. [PubMed] [Cross Ref]38.
Lunet N., Lacerda-Vieira A., Barros H. Fruit and vegetables consumption and gastric cancer: a systematic review and meta-analysis of cohort studies. Nutrition and Cancer. 2005;53(1):1–10. doi: 10.1207/s15327914nc5301_1. [PubMed] [Cross Ref]39.
Takezaki T., Gao C.-M., Wu J.-Z., et al. Dietary protective and risk factors for esophageal and stomach cancers in a low-epidemic area for stomach cancer in Jiangsu Province, China: comparison with those in a high-epidemic area. Japanese Journal of Cancer Research. 2001;92(11):1157–1165. doi: 10.1111/j.1349-7006.2001.tb02135.x. [PubMed] [Cross Ref]40.
Joossens J. V., Hill M. J., Elliott P., et al. Dietary salt, nitrate and stomach cancer mortality in 24 countries. European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. International Journal of Epidemiology. 1996;25(3):494–504. [PubMed]41.
Gonzalez C. A., Riboli E. Diet and cancer prevention: where we are, where we are going. Nutrition and Cancer. 2006;56(2):225–231. doi: 10.1207/s15327914nc5602_14. [PubMed] [Cross Ref]42.
Pakseresht M., Forman D., Malekzadeh R., et al. Dietary habits and gastric cancer risk in north-west Iran. Cancer Causes and Control. 2011;22(5):725–736. doi: 10.1007/s10552-011-9744-5. [PubMed] [Cross Ref]43.
Nouraie M., Pietinen P., Kamangar F., et al. Fruits, vegetables, and antioxidants and risk of gastric cancer among male smokers. Cancer Epidemiology Biomarkers and Prevention. 2005;14(9):2087–2092. doi: 10.1158/1055-9965.epi-05-0038. [PubMed] [Cross Ref]44.
Botterweck A. A. M., van den Brandt P. A., Goldbohm R. A. Vitamins, carotenoids, dietary fiber, and the risk of gastric carcinoma: results from a prospective study after 6.3 years of follow-up. Cancer. 2000;88(4):737–748. doi: 10.1002/(sici)1097-0142(20000215)88:460;737::aid-cncr262;3.0.co;2-h. [PubMed] [Cross Ref]45.
D'Elia L., Rossi G., Ippolito R., Cappuccio F. P., Strazzullo P. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clinical Nutrition. 2012;31(4):489–498. doi: 10.1016/j.clnu.2012.01.003. [PubMed] [Cross Ref]46.
Kneller R. W., Guo W.-D., Hsing A. W., et al. Risk factors for stomach cancer in sixty-five Chinese counties. Cancer Epidemiology Biomarkers and Prevention. 1992;1(2):113–118. [PubMed]47.
Fox J. G., Rogers A. B., Ihrig M., et al. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Research. 2003;63(5):942–950. [PubMed]48.
Rogers A. B., Taylor N. S., Whary M. T., Stefanich E. D., Wang T. C., Fox J. G. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Research. 2005;65(23):10709–10715. doi: 10.1158/0008-5472.CAN-05-1846. [PubMed] [Cross Ref]49.
Fox J. G., Dangler C. A., Taylor N. S., King A., Koh T. J., Wang T. C. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Research. 1999;59(19):4823–4828. [PubMed]50.
Gaddy J. A., Radin J. N., Loh J. T., et al. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infection and Immunity. 2013;81(6):2258–2267. doi: 10.1128/iai.01271-12. [PMC free article] [PubMed] [Cross Ref]51.
Loh J. T., Torres V. J., Cover T. L. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Research. 2007;67(10):4709–4715. doi: 10.1158/0008-5472.can-06-4746. [PubMed] [Cross Ref]52.
Voss B. J., Loh J. T., Hill S., Rose K. L., Mcdonald W. H., Cover T. L. Alteration of the Helicobacter pylori membrane proteome in response to changes in environmental salt concentration. Proteomics—Clinical Applications. 2015;9(11-12):1021–1034. doi: 10.1002/prca.201400176. [PMC free article] [PubMed] [Cross Ref]53.
Testerman T. L., Conn P. B., Mobley H. L. T., McGee D. J. Nutritional requirements and antibiotic resistance patterns of Helicobacter species in chemically defined media. Journal of Clinical Microbiology. 2006;44(5):1650–1658. doi: 10.1128/JCM.44.5.1650-1658.2006. [PMC free article] [PubMed] [Cross Ref]54.
Choby J. E., Skaar E. P. Heme synthesis and acquisition in bacterial pathogens. Journal of Molecular Biology. 2016 doi: 10.1016/j.jmb.2016.03.018. [PMC free article] [PubMed] [Cross Ref]55.
Skaar E. P., Raffatellu M. Metals in infectious diseases and nutritional immunity. Metallomics. 2015;7(6):926–928. doi: 10.1039/c5mt90021b. [PubMed] [Cross Ref]56.
Haley K. P., Skaar E. P. A battle for iron: host sequestration and Staphylococcus aureus acquisition. Microbes and Infection. 2012;14(3):217–227. doi: 10.1016/j.micinf.2011.11.001. [PMC free article] [PubMed] [Cross Ref]57.
Morgenthau A., Pogoutse A., Adamiak P., Moraes T. F., Schryvers A. B. Bacterial receptors for host transferrin and lactoferrin: molecular mechanisms and role in host-microbe interactions. Future Microbiology. 2013;8(12):1575–1585. doi: 10.2217/fmb.13.125. [PubMed] [Cross Ref]58.
Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102(3):783–788. doi: 10.1182/blood-2003-03-0672. [PubMed] [Cross Ref]59.
Senkovich O., Ceaser S., McGee D. J., Testerman T. L. Unique host iron utilization mechanisms of Helicobacter pylori revealed with iron-deficient chemically defined media. Infection and Immunity. 2010;78(5):1841–1849. doi: 10.1128/IAI.01258-09. [PMC free article] [PubMed] [Cross Ref]60.
Danielli A., Romagnoli S., Roncarati D., Costantino L., Delany I., Scarlato V. Growth phase and metal-dependent transcriptional regulation of the fecA genes in Helicobacter pylori. Journal of Bacteriology. 2009;191(11):3717–3725. doi: 10.1128/jb.01741-08. [PMC free article] [PubMed] [Cross Ref]61.
Ernst F. D., Bereswill S., Waidner B., et al. Transcriptional profiling of Helicobacter pylori Fur- and iron-regulated gene expression. Microbiology. 2005;151, part 2:533–546. doi: 10.1099/mic.0.27404-0. [PubMed] [Cross Ref]62.
Delany I., Pacheco A. B. F., Spohn G., Rappuoli R., Scarlato V. Iron-dependent transcription of the frpB gene of Helicobacter pylori is controlled by the Fur repressor protein. Journal of Bacteriology. 2001;183(16):4932–4937. doi: 10.1128/jb.183.16.4932-4937.2001. [PMC free article] [PubMed] [Cross Ref]63.
Pich O. Q., Carpenter B. M., Gilbreath J. J., Merrell D. S. Detailed analysis of Helicobacter pylori Fur-regulated promoters reveals a Fur box core sequence and novel Fur-regulated genes. Molecular Microbiology. 2012;84(5):921–941. doi: 10.1111/j.1365-2958.2012.08066.x. [PMC free article] [PubMed] [Cross Ref]64.
Vannini A., Roncarati D., Spinsanti M., Scarlato V., Danielli A. In depth analysis of the Helicobacter pylori cag pathogenicity island transcriptional responses. PLoS ONE. 2014;9(6) doi: 10.1371/journal.pone.0098416.e98416 [PMC free article] [PubMed] [Cross Ref]65.
Haley K. P., Blanz E. J., Gaddy J. A. High resolution electron microscopy of the Helicobacter pylori cag type IV secretion system pili produced in varying conditions of iron availability. Journal of Visualized Experiments. 2014;(93) doi: 10.3791/52122.e52122 [PMC free article] [PubMed] [Cross Ref]66.
Noto J. M., Gaddy J. A., Lee J. Y., et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. The Journal of Clinical Investigation. 2013;123(1):479–492. doi: 10.1172/jci64373. [PMC free article] [PubMed] [Cross Ref]67.
Tan S., Noto J. M., Romero-Gallo J., Peek R. M., Jr., Amieva M. R. Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS Pathogens. 2011;7(5, article e1002050) doi: 10.1371/journal.ppat.1002050. [PMC free article] [PubMed] [Cross Ref]68.
Yuan Y., Wu Q., Cheng G., et al. Recombinant human lactoferrin enhances the efficacy of triple therapy in mice infected with Helicobacter pylori. International Journal of Molecular Medicine. 2015;36(2):363–368. [PMC free article] [PubMed]69.
Akiba S., Neriishi K., Blot W. J., et al. Serum ferritin and stomach cancer risk among a Japanese population. Cancer. 1991;67(6):1707–1712. doi: 10.1002/1097-0142(19910315)67:660;1707::aid-cncr282067063862;3.0.co;2-c. [PubMed] [Cross Ref]70.
Nomura A., Chyou P.-H., Stemmermann G. N. Association of serum ferritin levels with the risk of stomach cancer. Cancer Epidemiology Biomarkers and Prevention. 1992;1(7):547–550. [PubMed]71.
Harrison L. E., Zhang Z.-F., Karpeh M. S., Sun M., Kurtz R. C. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: a Case-Control Study in the U.S. Cancer. 1997;80(6):1021–1028. doi: 10.1002/(sici)1097-0142(19970915)80:6<1021::aid-cncr3>3.0.co;2-c. [PubMed] [Cross Ref]72.
Haley K. P., Delgado A. G., Piazuelo M. B., et al. The human antimicrobial protein calgranulin C participates in control of Helicobacter pylori growth and regulation of virulence. Infection and Immunity. 2015;83(7):2944–2956. doi: 10.1128/iai.00544-15. [PMC free article] [PubMed] [Cross Ref]73.
Gaddy J. A., Radin J. N., Loh J. T., et al. The host protein calprotectin modulates the Helicobacter pylori cag Type IV secretion system via zinc sequestration. PLoS Pathogens. 2014;10(10) doi: 10.1371/journal.ppat.1004450.e1004450 [PMC free article] [PubMed] [Cross Ref]74.
Sempértegui F., Díaz M., Mejía R., et al. Low concentrations of zinc in gastric mucosa are associated with increased severity of Helicobacter pylori-induced inflammation. Helicobacter. 2007;12(1):43–48. doi: 10.1111/j.1523-5378.2007.00476.x. [PubMed] [Cross Ref]75.
Gaddy J. A., Radin J. N., Cullen T. W., et al. Helicobacter pylori resists the antimicrobial activity of calprotectin via lipid A modification and associated biofilm formation. mBio. 2015;6(6) doi: 10.1128/mbio.01349-15.e01349-15 [PMC free article] [PubMed] [Cross Ref]76.
Dawsey S. P., Hollenbeck A., Schatzkin A., Abnet C. C. A prospective study of vitamin and mineral supplement use and the risk of upper gastrointestinal cancers. PLoS ONE. 2014;9(2, article e88774) doi: 10.1371/journal.pone.0088774. [PMC free article] [PubMed] [Cross Ref]77.
Toyonaga A., Okamatsu H., Sasaki K., et al. Epidemiological study on food intake and Helicobacter pylori infection. Kurume Medical Journal. 2000;47(1):25–30. doi: 10.2739/kurumemedj.47.25. [PubMed] [Cross Ref]78.
Janjetic M. A., Goldman C. G., Balcarce N. E., et al. Iron, zinc, and copper nutritional status in children infected with Helicobacter pylori. Journal of Pediatric Gastroenterology and Nutrition. 2010;51(1):85–89. doi: 10.1097/mpg.0b013e3181c2c2cd. [PubMed] [Cross Ref]79.
Baik S. J., Yi S. Y., Park H. S., Park B. H. Seroprevalence of Helicobacter pylori in female Vietnamese immigrants to Korea. World Journal of Gastroenterology. 2012;18(6):517–521. doi: 10.3748/wjg.v18.i6.517. [PMC free article] [PubMed] [Cross Ref]80.
Herrmann L., Schwan D., Garner R., et al. Helicobacter pylori cadA encodes an essential Cd(II)-Zn(II)-Co(II) resistance factor influencing urease activity. Molecular Microbiology. 1999;33(3):524–536. doi: 10.1046/j.1365-2958.1999.01496.x. [PubMed] [Cross Ref]81.
Zambelli B., Turano P., Musiani F., Neyroz P., Ciurli S. Zn2+-linked dimerization of UreG from Helicobacter pylori, a chaperone involved in nickel trafficking and urease activation. Proteins: Structure, Function and Bioinformatics. 2009;74(1):222–239. doi: 10.1002/prot.22205. [PubMed] [Cross Ref]82.
Sydor A. M., Lebrette H., Ariyakumaran R., Cavazza C., Zamble D. B. Relationship between Ni(II) and Zn(II) coordination and nucleotide binding by the helicobacter pylori -hydrogenase and urease maturation factor HypB. Journal of Biological Chemistry. 2014;289(7):3828–3841. doi: 10.1074/jbc.M113.502781. [PMC free article] [PubMed] [Cross Ref]83.
Bellucci M., Zambelli B., Musiani F., Turano P., Ciurli S. Helicobacter pylori UreE, a urease accessory protein: Specific Ni2+- and Zn2+-binding properties and interaction with its cognate UreG. Biochemical Journal. 2009;422(1):91–100. doi: 10.1042/bj20090434. [PubMed] [Cross Ref]84.
Johnson R. C., Hu H. Q., Merrell D. S., Maroney M. J. Dynamic HypA zinc site is essential for acid viability and proper urease maturation in Helicobacter pylori. Metallomics. 2015;7(4):674–682. doi: 10.1039/c4mt00306c. [PMC free article] [PubMed] [Cross Ref]85.
Xia W., Li H., Sze K.-H., Sun H. Structure of a nickel chaperone, HypA, from Helicobacter pylori reveals two distinct metal binding sites. Journal of the American Chemical Society. 2009;131(29):10031–10040. doi: 10.1021/ja900543y. [PubMed] [Cross Ref]86.
Herbst R. W., Perovic I., Martin-Diaconescu V., et al. Communication between the zinc and nickel sites in dimeric HypA: metal recognition and pH sensing. Journal of the American Chemical Society. 2010;132(30):10338–10351. doi: 10.1021/ja1005724. [PMC free article] [PubMed] [Cross Ref]87.
Waidner B., Melchers K., Stähler F. N., Kist M., Bereswill S. The Helicobacter pylori CrdRS two-component regulation system (HP1364/HP1365) is required for copper-mediated induction of the copper resistance determinant CrdA. Journal of Bacteriology. 2005;187(13):4683–4688. doi: 10.1128/jb.187.13.4683-4688.2005. [PMC free article] [PubMed] [Cross Ref]88.
Waidner B., Melchers K., Ivanov I., et al. Identification by RNA profiling and mutational analysis of the novel copper resistance determinants CrdA (HP1326), CrdB (HP1327), and CzcB (HP1328) in Helicobacter pylori. Journal of Bacteriology. 2002;184(23):6700–6708. doi: 10.1128/jb.184.23.6700-6708.2002. [PMC free article] [PubMed] [Cross Ref]89.
Stähler F. N., Odenbreit S., Haas R., et al. The novel Helicobacter pylori CznABC metal efflux pump is required for cadmium, zinc, and nickel resistance, urease modulation, and gastric colonization. Infection and Immunity. 2006;74(7):3845–3852. doi: 10.1128/iai.02025-05. [PMC free article] [PubMed] [Cross Ref]90.
Tran C. D., Campbell M. A. F., Kolev Y., Chamberlain S., Huynh H. Q., Butler R. N. Short-term zinc supplementation attenuates Helicobacter felis-induced gastritis in the mouse. Journal of Infection. 2005;50(5):417–424. doi: 10.1016/j.jinf.2004.07.008. [PubMed] [Cross Ref]91.
Picot J., Hartwell D., Harris P., Mendes D., Clegg A. J., Takeda A. The effectiveness of interventions to treat severe acute malnutrition in young children: a systematic review. Health Technology Assessment. 2012;16(19):1–316. doi: 10.3310/hta16190. [PMC free article] [PubMed] [Cross Ref]92.
Campanale M., Nucera E., Ojetti V., et al. Nickel free-diet enhances the Helicobacter pylori eradication rate: a pilot study. Digestive Diseases and Sciences. 2014;59(8):1851–1855. doi: 10.1007/s10620-014-3060-3. [PubMed] [Cross Ref]93.
MacOmber L., Hausinger R. P. Mechanisms of nickel toxicity in microorganisms. Metallomics. 2011;3(11):1153–1162. doi: 10.1039/c1mt00063b. [PMC free article] [PubMed] [Cross Ref]94.
de Reuse H., Vinella D., Cavazza C. Common themes and unique proteins for the uptake and trafficking of nickel, a metal essential for the virulence of Helicobacter pylori. Frontiers in Cellular and Infection Microbiology. 2013;3, article 94 doi: 10.3389/fcimb.2013.00094. [PMC free article] [PubMed] [Cross Ref]95.
Fulkerson J. F., Jr., Mobley H. L. Membrane topology of the NixA nickel transporter of Helicobacter pylori: two nickel transport-specific motifs within transmembrane helices II and III. Journal of Bacteriology. 2000;182(6):1722–1730. [PMC free article] [PubMed]96.
Schauer K., Gouget B., Carrière M., Labigne A., De Reuse H. Novel nickel transport mechanism across the bacterial outer membrane energized by the TonB/ExbB/ExbD machinery. Molecular Microbiology. 2007;63(4):1054–1068. doi: 10.1111/j.1365-2958.2006.05578.x. [PubMed] [Cross Ref]97.
Stoof J., Kuipers E. J., van Vliet A. H. M. Characterization of NikR-responsive promoters of urease and metal transport genes of Helicobacter mustelae. BioMetals. 2010;23(1):145–159. doi: 10.1007/s10534-009-9275-7. [PubMed] [Cross Ref]98.
Stoof J., Kuipers E. J., Klaver G., Van Vliet A. H. M. An ABC transporter and a TonB ortholog contribute to Helicobacter mustelae nickel and cobalt acquisition. Infection and Immunity. 2010;78(10):4261–4267. doi: 10.1128/iai.00365-10. [PMC free article] [PubMed] [Cross Ref]99.
Aebischer T., Fischer A., Walduck A., et al. Vaccination prevents Helicobacter pylori-induced alterations of the gastric flora in mice. FEMS Immunology and Medical Microbiology. 2006;46(2):221–229. doi: 10.1111/j.1574-695x.2005.00024.x. [PubMed] [Cross Ref]100.
Yin Y.-N., Wang C.-L., Liu X.-W., et al. Gastric and duodenum microflora analysis after long-term Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2011;16(5):389–397. doi: 10.1111/j.1523-5378.2011.00862.x. [PubMed] [Cross Ref]101.
Osaki T., Matsuki T., Asahara T., et al. Comparative analysis of gastric bacterial microbiota in Mongolian gerbils after long-term infection with Helicobacter pylori. Microbial Pathogenesis. 2012;53(1):12–18. doi: 10.1016/j.micpath.2012.03.008. [PubMed] [Cross Ref]102.
Sun Y.-Q., Monstein H.-J., Nilsson L. E., Petersson F., Borch K. Profiling and identification of eubacteria in the stomach of Mongolian gerbils with and without Helicobacter pylori infection. Helicobacter. 2003;8(2):149–157. doi: 10.1046/j.1523-5378.2003.00136.x. [PubMed] [Cross Ref]103.
Maldonado-Contreras A., Goldfarb K. C., Godoy-Vitorino F., et al. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME Journal. 2011;5(4):574–579. doi: 10.1038/ismej.2010.149. [PMC free article] [PubMed] [Cross Ref]104.
Aviles-Jimenez F., Vazquez-Jimenez F., Medrano-Guzman R., Mantilla A., Torres J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Scientific Reports. 2014;4, article 4202 doi: 10.1038/srep04202. [PMC free article] [PubMed] [Cross Ref]105.
Lee C.-W., Rickman B., Rogers A. B., Ge Z., Wang T. C., Fox J. G. Helicobacter pylori eradication prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer Research. 2008;68(9):3540–3548. doi: 10.1158/0008-5472.CAN-07-6786. [PMC free article] [PubMed] [Cross Ref]106.
Lofgren J. L., Whary M. T., Ge Z., et al. Lack of commensal flora in helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology. 2011;140(1):210–220. doi: 10.1053/j.gastro.2010.09.048. [PMC free article] [PubMed] [Cross Ref]
Articles from Gastroenterology Research and Practice are provided here courtesy of Hindawi Publishing Corporation
Probiotic supplementation can reduce the antibiotic-induced alteration and imbalance of the gut microbiota composition. This effect may restrict the growth of antibiotic-resistant bacteria in the gut and improve the Helicobacter pylori eradication success rate.
The Effect of Probiotics on Gut Microbiota during the Helicobacter pylori Eradication: Randomized Controlled Trial
- Bumjo Oh1,†,
- Bong-Soo Kim2,†,
- Ji Won Kim3,*,
- Jong Seung Kim1,
- Seong-Joon Koh3,
- Byeong Gwan Kim3,
- Kook Lae Lee3 and
- Jongsik Chun4,5
Version of Record online: 23 SEP 2015
DOI: 10.1111/hel.12270
© 2015 John Wiley & Sons Ltd
Abstract
Background
Helicobacter pylori causes chronic gastritis, gastroduodenal ulcers, and gastric cancer, and has been treated with two antibiotics (amoxicillin and clarithromycin) and proton-pump inhibitors (PPIs). However, antibiotic treatment alters the indigenous gut microbiota to cause side effects. Therefore, the effects of probiotic supplementation on therapy have been studied. Although several studies have covered the probiotics’ effects, details about the gut microbiota changes after H. pylori eradication have not been evaluated. Therefore, we analyzed the influences of antibiotics and their combination with probiotics on the composition of the gut microbiota using high-throughput sequencing.
Methods
Subjects were divided into two groups. The antibiotics group was treated with general therapy, and the probiotics group with general therapy and probiotic supplementation. Fecal samples were collected from all subjects during treatments, and the influences on gut microbiota were analyzed by 16S rRNA gene-pyrosequencing.
Results
Three phyla, Firmicutes, Bacteroidetes, and Proteobacteria, were predominant in the gut microbiota of all subjects. After treatment, the relative abundances of Firmicutes were reduced, whereas those of Proteobacteria were increased in both groups. However, the changed proportions of the gut microbiota in the antibiotics group were higher than those in the probiotics group. In addition, the increase in the levels of antibiotic-resistant bacteria was higher in the antibiotics group than in the probiotics one.
Conclusion
Probiotic supplementation can reduce the antibiotic-induced alteration and imbalance of the gut microbiota composition. This effect may restrict the growth of antibiotic-resistant bacteria in the gut and improve the H. pylori eradication success rate.
probiotica, Clostridium difficile infectie, antibiotica, infecties, maagzweer, Helicobacter pylori, voeding en voedingsstoffen, groene thee
Gerelateerde artikelen
 H. Pylori en maagkanker
H. Pylori en maagkanker



Plaats een reactie ...
Reageer op "Helicobacter Pylori bacterie: Voeding en voedingsstoffen kan de bacterie Helicobacter pylori voorkomen en elimineren en daarmee een maagzweer en maagkanker voorkomen"