20 november 2023: Bron: BLOOD conferentie
Blinatumomab, een vorm van immuuntherapie met T-cellen aanvullend op chemotherapie heeft bewezen effectief te zijn in zowel minimale ziekte als bij een recidief van Acute Lymfatische Leukemie - All bij zowel babies, kinderen als ook bij volwassenen.
Uit een nieuwe studie blijkt Blinatumomab ook uitstekende resultaten te geven aanvullend op chemotherapie met lage dosis bij volwassenen in de leeftijd van 40 tot 65 jaar.
De onderzoekers ontdekten dat minimale residuele ziekte (MRD) werd bereikt door 70 procent van de patiënten aan het einde van cyclus 1B en 83 procent van de patiënten aan het einde van cyclus 2B. De geschatte recidiefvrije overleving na 24 maanden was 60,4 procent, en de totale overleving (OS) na 24 maanden was 78,6 procent.
In deze grafiek (Figure 1) wordt de werking van Blinatumomab uitgelegd:
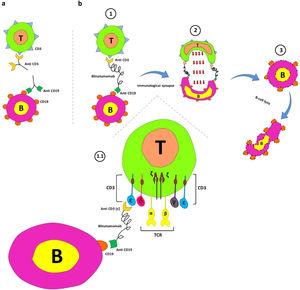
Figure 1.
Blinatumomab's mechanism of action. a. Interaction between anti-CD19 and CD19 on B cells and anti-CD3 and CD3 on T cells. b. The main components of the blinatumomab (two recombinant single-chain variable fragments of anti-CD3 and anti-CD19) and its mechanism of action; 1. Transient engagement of B cells and T cells by blinatumomab; 1.1. Anti-CD3 links ε chain of CD3 on T-cells anti-CD19 connects CD19 on B cells; 2. Immunological synapse formation and releasing of serine proteases (such as perforins and granzymes) by activated T cells, and; 3. B cell apoptosis.
“Oudere volwassenen met ALL hebben een aanzienlijk slechtere uitkomst dan hun jongere leeftijdsgenoten, als gevolg van een combinatie van hogere percentages ziektes met een laag risico en een slechtere tolerantie voor intensieve therapieën,” aldus Shaun Fleming, M.B.B.S., van Alfred Health en Monash University in Melbourne, Australië. “We hebben de studie opgezet om blinatumomab te implementeren, waarvan de werkzaamheid is bewezen bij recidiverende/refractaire en MRD-positieve ALL, in combinatie met chemotherapie met verminderde intensiteit om te proberen de ziekterespons te optimaliseren en de toxiciteit te verminderen.”
Fleming en collega's evalueerden gegevens van 30 patiënten in de leeftijd van 40 tot 65 jaar met B-celvoorloper ALL. Na een steroïde prefase en debulking-chemotherapie ontvingen de patiënten vier afwisselende cycli van Blinatumomab en hyper-CVAD-chemotherapie. Degenen met een ziekte met een hoog risico werd aanbevolen een allogene stamceltransplantatie te ondergaan, en anderen kregen een POMP-onderhoudstherapie.
“De bevindingen van deze studie tonen de haalbaarheid aan van het combineren van blinatumomab met chemotherapie met lage intensiteit voor oudere volwassenen met B-ALL. De combinatie rechtvaardigt verder onderzoek en heeft geleid tot het ontwerp van gerandomiseerde klinische onderzoeken bij deze patiëntenpopulatie.”, aldus Shaun Fleming tijdens de presentatie op de Blood conferentie.
In het abstractenboek van BLOOD 2023 staat deze studie vermeld vanaf pagina 149 en de presentatie op bladzijde 49.
Onder de volgende titel staan bijna alle studies met blinatumab vermeld die tot nu toe zijn uitgevoerd of nog lopen:
B cell acute lymphoblastic leukemia-lymphoma (B-ALL) accounts for approximately 75% of ALL cases and is observed in children and adults. Recent advances in disease diagnosis, stratification and prognostication have led to a better characterization of different subgroups of ALL. Notwithstanding the significant improvement in the complete remission rate of B-ALL, patients with minimal residual disease (MRD) and relapsed/refractory (R/R) settings suffer from poor outcomes. Hypothesis: However, novel therapies, such as agents targeting tyrosine kinases or the CD20 molecule, combination therapies and improved supportive care, have changed the treatment landscape of B-ALL.
Method and resultsMeanwhile, blinatumomab has been FDA-approved for MRD-positive or R/R B-ALL patients. Blinatumomab is a bispecific T cell engager containing the CD3 and CD19 that recognize domains redirecting cytotoxic T cells to lyse B cells. Promising outcomes, including long-term overall survival and improved MRD-negative response rates, have been reported in patients who received this drug. Adding blinatumomab to new ALL regimens seems promising for achieving better outcomes in poor prognosis B-ALL patients. Nevertheless, the neurotoxicity and cytokine release syndrome are the two major adverse events following the blinatumomab therapy.
ConclusionThis review summarizes the function and effectiveness of blinatumomab in R/R and MRD positive B-ALL patients. Furthermore, blinatumomab's positive and negative aspects as a novel therapy for B-ALL patients have been briefly discussed.
The advent and utilization of blinatumomab is evidence of shifting the old paradigm to the emerging plans for better B-ALL management (Table 1). A B-ALL cohort with 239 patients (relapsed refractory, n = 227, and MRD positive, n = 12) who received blinatumomab therapy evaluated the safety and efficacy of this approach in the real-world context. Before blinatumomab initiation, 26% (n = 61) patients had received ≥ 3 previous therapies and 19% (n = 46) had undergone HSCT. The CR/CRi rate (CR with incomplete hematologic recovery) was 65% in RR patients. Among MRD-positive patients who received blinatumomab, 75% achieved MRD negativity. RR patients who received blinatumomab showed median RFS and OS of 32 months and 13 months, respectively. In positive MRD patients, the median RFS and OS were not achieved. Neurotoxicity, hepatotoxicity and cytokine release syndrome (CRS) with grade ≥ 3 were reported in 7%, 10% and 3% of patients, respectively. The allo-HSCT, as consolidation therapy in patients with CR/Cri, retained its favorable prognostic effect for OS (p = 0.04).13,14
Clinical trials of blinatumomab in R/R B-ALL.
| Study | Results | Ref |
|---|---|---|
| Phase II trial |
|
22 |
| Phase II trial |
|
23 |
| Phase Ib |
|
24 |
| Phase II |
|
25 |
| Phase II |
|
26 |
| Phase III |
|
27 |
| Phase II |
|
28 |
| Randomized phase III |
|
29 |
| Retrospective multicenter study |
|
30 |
CR/CRh, Complete Remission/Complete Remission with Partial Hematologic Recovery; MRD, Minimal Residual Disease; RFS, Relapse-Free Survival; OS, Overall Survival; R/R B-cell precursor ALL, Relapsed/Refractory B-Cell Precursor Acute Lymphoblastic Leukemia; R/R Phþ ALL, Relapsed/Refractory Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia; SOC, Standard Of Care; allo-HSCT, Allogeneic-Hematopoietic Stem Cell Transplantation.
Advanced treatment strategies and novel drugs have improved the clinical outcome of B-cell ALL patients. For instance, tyrosine kinase inhibitors (target JAK/STAT and mTOR pathways), antibody drug-conjugate, bispecific antibodies and chimeric antigen receptor T cells are promising in achieving more favorable outcomes. However, the outcome in R/R ALL patients remains poor. The FDA has approved blinatumomab for R/R B-ALL and MRD+B-ALL. The poor prognosis ALL patients with some translocations, such as the TCF3/HLF-fusion and Philadelphia-positive or Philadelphia-like ALL are candidates to receive blinatumomab. single-agent blinatumomab and its combination with other therapies, such as TKIs have improved survival and long-term outcomes. Furthermore, combining checkpoint inhibitors (anti-PDL-1 and anti-CTLA-4 antibodies) with blinatumomab could enhance its effectiveness. Although blinatumomab administration is tolerable and safe, prevention and optimal management of blinatumomab-induced adverse reactions are considerable issues.
Data availability statementThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributionsB.H.K., S.P. and RM. were responsible for the conception and design and acquisition of data; H.G.N., M.M., drafting the article or revising it critically for important intellectual content, and; M.T.A., M.S. and M.D.G.H, methodology (lead), writing, review and editing. All authors revised the final approval of the version to be submitted for publication.
Gerelateerde artikelen
- blinatumomab met chemotherapie met lage dosis geeft uitstekende resultaten voor oudere volwassenen (40 plus) met B-cel Acute Lymfatische Leukemie.
- Blinatumomab geeft hele goede resultaten bij baby's met Acute Lymfatische Leukemie 93 vs 66 procent op 2-jaars meting, bewijst Nederlands onderzoek.
- Blinatumomab (Blincyto) geeft volledige minimale zogeheten residuele ziekte bij 80 procent van de patiënten met All - acute lymfatische leukemia en kan ALL genezen
- Blinatumomab, een nieuw gericht middel voor kankercellen met CD-19 expressie, zorgt in fase II studie bij 9 van de 12 deelnemers met uitbehandelde vergevorderde ALL, voor complete remissie.
- blinatumomab bij een recidief of ziekteprogressie van ALL - acute lymfatische leukemie blijkt uitstekend medicijn bij zowel kinderen als volwassenen blijkt uit reviewstudie



Plaats een reactie ...
Reageer op "blinatumomab met chemotherapie met lage dosis geeft uitstekende resultaten voor oudere volwassenen (40 plus) met B-cel Acute Lymfatische Leukemie."