Aan dit artikel is enkele uren gewerkt. Opzoeken, vertalen, plaatsen enz. Als u ons wilt ondersteunen dan kan dat via een al of niet anonieme donatie. Elk bedrag is welkom hoe klein ook. Klik hier als u ons wilt helpen kanker-actueel online te houden Wij zijn een ANBI organisatie en dus is uw donatie aftrekbaar voor de belasting.
26 juanuari 2019: Lees ook dit artikel: https://kanker-actueel.nl/dendritische-celtherapie-heeft-succes-bij-patienten-met-mesothelioma-asbestkanker-erasmus-doet-oproep-dat-meer-patienten-zich-aanmelden-voor-de-studie.html
1 april 2017: Lees ook ditt artikel:
https://kanker-actueel.nl/NL/pembrolizumab-een-anti-pd-medicijn-geeft-uitstekende-resultaten-bij-zwaar-voorbehandelde-patienten-met-mesothelioma.html
1 april 2017: lees ook dit studierapport, een meta analyse bij mesothelioma - asbestkanker: Diagnostic value of microRNAs in asbestos exposure and malignant mesothelioma: systematic review and qualitative meta-analysis over welke aanpak bij mesothelioma eventueel zou kunnen werken. Met ook hier een interessante referentielijst. Tekst gaat onder beeld verder.
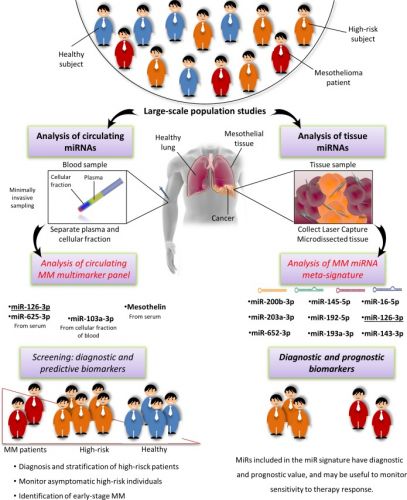
Schematic drawing illustrating summary findings
En lees onderstaand artikel over ranpirnasse.
Ook in dit studierapport met interessante referentielijst: Trop-2-targeting tetrakis-ranpirnase has potent antitumor activity against triple-negative breast cancer bewijst tetrakis-ranpirnase ook effectiviteit bij triple negatieve borstkanker. In alle studies met ranpirnase komt naar voren dat dit middel werkt bij bepaalde mutaties en tumorenexpressie TROP-2 expressie. Er is best veel ondezoek naar gedaan, zie ook referentielijst onderaan dit artikel. En heel belangrijk het lijkt een medicijn te zijn dat weinig bijwerkingen geeft.
18 januari 2012:
Nu Nederlandse onderzoekers hebben aangetoond dat een chemo behandeling bij zowel buik mesothelioma als long mesothelioma geen positief effect hebben op de mediane overleving is de aanpak met niet toxische enzymen onder de naam ranpirnase - onconase nog belangrijker geworden. Wie hier klikt kan een volledig studierapport inzien over ranprinase - onconase bij mesothelioma dat bijzonder positief oordeelt over dit niet toxische middel. Hier de conclusie van deze review en daaronder een studie abstract met ranpirnasse uit 2002 met bijzonder goede resultaten op de mediane overlevingstijd. Mocht u mesothelioma hebben neem dit studierapport aub mee naar uw oncoloog - arts.
Ranpirnase is a ribonuclease endowed with potent antitumor properties; its mechanism of action is completely novel since, by degrading tRNA, it acts both as a cytotoxic and a cytostatic drug; furthermore, owing to both its in vitro synergy with other cytotoxic agents, and its tolerability as a single agent, it is amenable to combination with traditional chemothera-peutic drugs, eg, doxorubicin and cisplatin.
Since its early clinical development, it has held great promise for the treatment of MMe, where it seems to act mainly as a cytostatic agent, thus stabilizing the disease. Indeed, ranpirnase proved to be superior to doxorubicin within a phase III trial, while preliminary results of another large, phase III trial suggest that the combination of ranpirnase and doxorubicin could be more effective than doxorubicin alone
Ranpirnase appears to be generally well tolerated with predicable and reversible toxicity, and with very few serious adverse events; furthermore, dose modifications are usually required only for changes in renal function.
Unfortunately for the development of the drug, during the course of its phase III development, the combination of pemetrexed and a platinum derivative emerged as the standard first-line treatment for MMe patients in. This led to a slowing of the recruitment into the P30–302 protocol and clearly reduced the interest for its use as a first-line treatment.
Thus, while waiting the final results of the above study, it appears clear that ranpirnase may find its niche (in combination with doxorubicin) for second-line therapy of MMe (Pavlakis and Vogelzang 2006) where, at present, no standard of care exists.
However, the current understanding of its mechanism of action, coupled with its favorable toxicity profile, characterized by a lack of major toxicities, especially in terms of hematology, make ranpirnase an appealing drug to use in combination with other anticancer agents, as well as with radiotherapy. This could clearly open a new frontier for the use of this novel drug in tumor types other than MMe.
2002:
In een Phase II trial bleek het medicijn ranpirnase (onconase) maar liefst bij 41 van de 81 deelnemende patiënten de tumorgroei tot stilstand te brengen. De overlevingstijd werd verlengd tot gemiddeld 18,5 maanden, een verbetering van 6-8 maanden in vergelijking met de overlevingtijd die normaal staat voor een mesothelioma patiënt. Dit resultaat was nog opmerkelijker omdat een derde van de patiënten helemaal niet had gereageerd op eerdere chemobehandeling. Ranpirnase wordt gehaald uit de eieren van kikkers en de patiënten vertoonden geen bijwerkingen zoals normaal gesproken voorkomen bij een behandeling met bv. chemo.
A promising drug for mesothelioma
Results of a Phase II multicentre trial indicate that the ribonuclease-based drug ranpirnase (Onconase) shows promise as a treatment for patients with inoperable malignant mesothelioma. This asbestos-related cancer of the inner lining of the chest and abdomen will kill over 250,000 people in Europe alone in the next 35 years. As reported in the Journal of Clinical Oncology, the tumours either shrank or stopped growing in 41 of the 81 patients that were assessable for tumour response. The median survival time for these patients was 18.5 months — a dramatic improvement over the 6–8-month life expectancy for the average mesothelioma patient. The results were especially encouraging in light of the fact that over one-third of these patients did not respond successfully to prior systemaic therapy. Ranpirnase — a ribonuclease that was developed from the eggs of the frog, Rana pipiens — interrupts protein synthesis, resulting in the inhibition of cell growth and the induction of apoptosis in cancer cells. The drug has been shown to be well tolerated in most patients, and has not been associated with the toxicity that is typically associated with chemotherapy. A randomized, controlled Phase III trial of the combination of ranpirnase with doxorubicin in patients with inoperable malignant mesothelioma, compared with doxorubicin therapy alone, is also underway in the United States and Europe.
ORIGINAL RESEARCH PAPER
Mikulski, S. M. et al. Phase II trial of a single weekly intravenous dose of ranpirnase in patients with unresectable malignant mesothelioma. J. Clin. Oncol. 20, 274–281 (2002). | PubMed |
A new class of immunoRNases was generated with enhanced potency for targeted therapy of cancer. The promising results from (Rap)2-E1-(Rap)2 and (Rap)2-E1*-(Rap)2 support their further investigation as a potential treatment option for TNBC and other Trop-2-expressing cancers.
Trop-2-targeting tetrakis-ranpirnase has potent antitumor activity against triple-negative breast cancer
Donglin Liu,
 1,2 Thomas M Cardillo
1,2 Thomas M Cardillo,
2 Yang Wang,
2 Edmund A Rossi,
1,2 David M Goldenberg,
 1,2,3
1,2,3 and
Chien-Hsing Chang1,2
This article has been
cited by other articles in PMC.
Abstract
Background
Ranpirnase (Rap) is an amphibian ribonuclease with reported antitumor activity, minimal toxicity, and negligible immunogenicity in clinical studies, but the unfavorable pharmacokinetics and suboptimal efficacy hampered its further clinical development. To improve the potential of Rap-based therapeutics, we have used the DOCK-AND-LOCK™ (DNL™) method to construct a class of novel IgG-Rap immunoRNases. In the present study, a pair of these constructs, (Rap)2-E1-(Rap)2 and (Rap)2-E1*-(Rap)2, comprising four copies of Rap linked to the CH3 and CK termini of hRS7 (humanized anti-Trop-2), respectively, were evaluated as potential therapeutics for triple-negative breast cancer (TNBC).
Methods
The DNL-based immunoRNases, (Rap)2-E1-(Rap)2 and (Rap)2-E1*-(Rap)2, were characterized and tested for biological activities in vitro on a panel of breast cancer cell lines and in vivo in a MDA-MB-468 xenograft model.
Results
(Rap)2-E1-(Rap)2 was highly purified (>95%), exhibited specific cell binding and rapid internalization in MDA-MB-468, a Trop-2-expressing TNBC line, and displayed potent in vitro cytotoxicity (EC50 ≤ 1 nM) against diverse breast cancer cell lines with moderate to high expression of Trop-2, including MDA-MB-468, BT-20, HCC1806, SKBR-3, and MCF-7. In comparison, structural counterparts of (Rap)2-E1-(Rap)2, generated by substituting hRS7 with selective non-Trop-2-binding antibodies, such as epratuzumab (anti-CD22), were at least 50-fold less potent than (Rap)2-E1-(Rap)2 in MDA-MB-468 and BT-20 cells, both lacking the expression of the cognate antigen. Moreover, (Rap)2-E1-(Rap)2 was less effective (EC50 > 50 nM) in MDA-MB-231 (low Trop-2) or HCC1395 (no Trop-2), and did not show any toxicity to human peripheral blood mononuclear cells. In a mouse TNBC model, a significant survival benefit was achieved with (Rap)2-E1*-(Rap)2 when given the maximal tolerated dose.
Conclusions
A new class of immunoRNases was generated with enhanced potency for targeted therapy of cancer. The promising results from (Rap)2-E1-(Rap)2 and (Rap)2-E1*-(Rap)2 support their further investigation as a potential treatment option for TNBC and other Trop-2-expressing cancers.
References
- Hutchinson L. Breast cancer: challenges, controversies, breakthroughs. Nat Rev Clin Oncol. 2010;7:669–670. doi: 10.1038/nrclinonc.2010.192. [PubMed] [Cross Ref]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [PubMed] [Cross Ref]
- Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [PubMed] [Cross Ref]
- Johnston SR. New strategies in estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:1979–1987. doi: 10.1158/1078-0432.CCR-09-1823. [PubMed] [Cross Ref]
- Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9:16–32. [PubMed]
- Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121:3797–3803. doi: 10.1172/JCI57152. [PMC free article] [PubMed] [Cross Ref]
- Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16(Suppl 1):1–11. [PubMed]
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [PubMed] [Cross Ref]
- Irshad S, Ellis P, Tutt A. Molecular heterogeneity of triple-negative breast cancer and its clinical implications. Curr Opin Oncol. 2011;23:566–577. doi: 10.1097/CCO.0b013e32834bf8ae. [PubMed] [Cross Ref]
- Metzger-Filho O, Tutt A, de Azambuja E, Saini KS, Viale G, Loi S, Bradbury I, Bliss JM, Azim HA Jr, Ellis P, Di Leo A, Baselga J, Sotiriou C, Piccart-Gebhart M. Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol. 2012;30:1879–1887. doi: 10.1200/JCO.2011.38.2010. [PubMed] [Cross Ref]
- Santana-Davila R, Perez EA. Treatment options for patients with triple-negative breast cancer. J Hematol Oncol. 2010;3:42. doi: 10.1186/1756-8722-3-42. [PMC free article] [PubMed] [Cross Ref]
- Ardelt W, Mikulski SM, Shogen K. Amino acid sequence of an anti-tumor protein from Rana pipiens oocytes and early embryos. Homology to pancreatic ribonucleases. J Biol Chem. 1991;266:245–251. [PubMed]
- Darzynkiewicz Z, Carter SP, Mikulski SM, Ardelt WJ, Shogen K. Cytostatic and cytotoxic effects of Pannon (P-30 Protein), a novel anticancer agent. Cell Tissue Kinet. 1988;21:169–182. [PubMed]
- Mikulski SM, Ardelt W, Shogen K, Bernstein EH, Menduke H. Striking increase of survival of mice bearing M109 Madison carcinoma treated with a novel protein from amphibian embryos. J Natl Cancer Inst. 1990;82:151–153. [PubMed]
- Rodríguez M, Torrent G, Bosch M, Rayne F, Dubremetz JF, Ribó M, Benito A, Vilanova M, Beaumelle B. Intracellular pathway of Onconase that enables its delivery to the cytosol. J Cell Sci. 2007;120:1405–1411. doi: 10.1242/jcs.03427. [PubMed] [Cross Ref]
- Saxena SK, Sirdeshmukh R, Ardelt W, Mikulski SM, Shogen K, Youle RJ. Entry into cells and selective degradation of tRNAs by a cytotoxic member of the RNase A family. J Biol Chem. 2002;277:15142–15146. doi: 10.1074/jbc.M108115200. [PubMed] [Cross Ref]
- Iordanov MS, Ryabinina OP, Wong J, Dinh TH, Newton DL, Rybak SM, Magun BE. Molecular determinants of apoptosis induced by the cytotoxic ribonuclease onconase: evidence for cytotoxic mechanisms different from inhibition of protein synthesis. Cancer Res. 2000;60:1983–1994. [PubMed]
- Rybak SM, Pearson JW, Fogler WE, Volker K, Spence SE, Newton DL, Mikulski SM, Ardelt W, Riggs CW, Kung HF, Longo DL. Enhancement of vincristine cytotoxicity in drug-resistant cells by simultaneous treatment with onconase, an antitumor ribonuclease. J Natl Cancer Inst. 1996;88:747–753. doi: 10.1093/jnci/88.11.747. [PubMed] [Cross Ref]
- Michaelis M, Cinatl J, Anand P, Rothweiler F, Kotchetkov R, von Deimling A, Doerr HW, Shogen K, Cinatl J Jr. Onconase induces caspase-independent cell death in chemoresistant neuroblastoma cells. Cancer Lett. 2007;250(1):107–116. doi: 10.1016/j.canlet.2006.09.018. [PubMed] [Cross Ref]
- Reck MKM, Jassem J, Eschbach C, Kozielski J, Costanzi JJ, Gatzemeier U, Shogen K, von Pawel J. Randomized, multicenter phase III study of ranpirnase plus doxorubicin (DOX) versus DOX in patients with unresectable malignant mesothelioma (MM) J Clin Oncol. 2009;27:15s. doi: 10.1200/JCO.2008.21.7695. [Cross Ref]
- Mikulski SM, Costanzi JJ, Vogelzang NJ, McCachren S, Taub RN, Chun H, Mittelman A, Panella T, Puccio C, Fine R, Shogen K. Phase II trial of a single weekly intravenous dose of ranpirnase in patients with unresectable malignant mesothelioma. J Clin Oncol. 2002;20:274–281. doi: 10.1200/JCO.20.1.274. [PubMed] [Cross Ref]
- Mikulski S, Grossman A, Carter P, Shogen K, Costanzi J. Phase-I human clinical-trial of onconase(r) (p-30 protein) administered intravenously on a weekly schedule in cancer-patients with solid tumors. Int J Oncol. 1993;3:57–64. [PubMed]
- Stepan LP, Trueblood ES, Hale K, Babcook J, Borges L, Sutherland CL. Expression of Trop2 cell surface glycoprotein in normal and tumor tissues: potential implications as a cancer therapeutic target. J Histochem Cytochem. 2011;59:701–710. doi: 10.1369/0022155411410430. [PMC free article] [PubMed] [Cross Ref]
- Wang J, Day R, Dong Y, Weintraub SJ, Michel L. Identification of Trop-2 as an oncogene and an attractive therapeutic target in colon cancers. Mol Cancer Ther. 2008;7:280–285. doi: 10.1158/1535-7163.MCT-07-2003. [PubMed] [Cross Ref]
- Stoyanova T, Goldstein AS, Cai H, Drake JM, Huang J, Witte ON. Regulated proteolysis of Trop2 drives epithelial hyperplasia and stem cell self-renewal via beta-catenin signaling. Genes Dev. 2012;26:2271–2285. doi: 10.1101/gad.196451.112. [PMC free article] [PubMed] [Cross Ref]
- Huang H, Groth J, Sossey-Alaoui K, Hawthorn L, Beall S, Geradts J. Aberrant expression of novel and previously described cell membrane markers in human breast cancer cell lines and tumors. Clin Cancer Res. 2005;11:4357–4364. doi: 10.1158/1078-0432.CCR-04-2107. [PubMed] [Cross Ref]
- Fang YJ, Lu ZH, Wang GQ, Pan ZZ, Zhou ZW, Yun JP, Zhang MF, Wan DS. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int J Colorectal Dis. 2009;24:875–884. doi: 10.1007/s00384-009-0725-z. [PubMed] [Cross Ref]
- Ohmachi T, Tanaka F, Mimori K, Inoue H, Yanaga K, Mori M. Clinical significance of TROP2 expression in colorectal cancer. Clin Cancer Res. 2006;12:3057–3063. doi: 10.1158/1078-0432.CCR-05-1961. [PubMed] [Cross Ref]
- Fong D, Spizzo G, Gostner JM, Gastl G, Moser P, Krammel C, Gerhard S, Rasse M, Laimer K. TROP2: a novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol. 2008;21:186–191. [PubMed]
- Cubas R, Li M, Chen C, Yao Q. Trop2: a possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta. 2009;1796:309–314. [PubMed]
- Chang CH, Rossi EA, Goldenberg DM. The dock and lock method: a novel platform technology for building multivalent, multifunctional structures of defined composition with retained bioactivity. Clin Cancer Res. 2007;13(18 Pt 2):5586s–5591s. [PubMed]
- Rossi EA, Goldenberg DM, Cardillo TM, McBride WJ, Sharkey RM, Chang CH. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer targeting. Proc Natl Acad Sci U S A. 2006;103:6841–6846. doi: 10.1073/pnas.0600982103. [PMC free article] [PubMed] [Cross Ref]
- Rossi EA, Goldenberg DM, Cardillo TM, Stein R, Chang CH. CD20-targeted tetrameric interferon-alpha, a novel and potent immunocytokine for the therapy of B-cell lymphomas. Blood. 2009;114:3864–3871. doi: 10.1182/blood-2009-06-228890. [PMC free article] [PubMed] [Cross Ref]
- Chang CH, Gupta P, Michel R, Loo M, Wang Y, Cardillo TM, Goldenberg DM. Ranpirnase (frog RNase) targeted with a humanized, internalizing, anti-Trop-2 antibody has potent cytotoxicity against diverse epithelial cancer cells. Mol Cancer Ther. 2010;9:2276–2286. doi: 10.1158/1535-7163.MCT-10-0338. [PubMed] [Cross Ref]
- Rossi EA, Chang CH, Cardillo TM, Goldenberg DM. Optimization of multivalent bispecific antibodies and immunocytokines with improved in vivo properties. Bioconjug Chem. 2013;24:63–71. doi: 10.1021/bc300488f. [PubMed] [Cross Ref]
- Newton DL, Hansen HJ, Mikulski SM, Goldenberg DM, Rybak SM. Potent and specific antitumor effects of an anti-CD22-targeted cytotoxic ribonuclease: potential for the treatment of non-Hodgkin lymphoma. Blood. 2001;97:528–535. doi: 10.1182/blood.V97.2.528. [PubMed] [Cross Ref]
- Chang CH, Sapra P, Vanama SS, Hansen HJ, Horak ID, Goldenberg DM. Effective therapy of human lymphoma xenografts with a novel recombinant ribonuclease/anti-CD74 humanized IgG4 antibody immunotoxin. Blood. 2005;106:4308–4314. doi: 10.1182/blood-2005-03-1033. [PubMed] [Cross Ref]
- Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Goldenberg DM. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res. 2011;17:3157–3169. doi: 10.1158/1078-0432.CCR-10-2939. [PubMed] [Cross Ref]
- Rossi EA, Goldenberg DM, Cardillo TM, Stein R, Wang Y, Chang CH. Novel designs of multivalent anti-CD20 humanized antibodies as improved lymphoma therapeutics. Cancer Res. 2008;68:8384–8392. doi: 10.1158/0008-5472.CAN-08-2033. [PubMed] [Cross Ref]
- Rossi DL, Rossi EA, Goldenberg DM, Chang CH. A new mammalian host cell with enhanced survival enables completely serum-free development of high-level protein production cell lines. Biotechnol Prog. 2011;27:766–775. doi: 10.1002/btpr.584. [PubMed] [Cross Ref]
Articles from Molecular Cancer are provided here courtesy of BioMed Central
The qualitative meta-analysis and functional investigation confirmed the early diagnostic value of two miRNA signatures for MM. Large-scale, standardized validation studies are needed to assess their clinical relevance, so as to move from the workbench to the clinic.
Diagnostic value of microRNAs in asbestos exposure and malignant mesothelioma: systematic review and qualitative meta-analysis
This article has been
cited by other articles in PMC.
Abstract
Background
Asbestos is a harmful and exceptionally persistent natural material. Malignant mesothelioma (MM), an asbestos-related disease, is an insidious, lethal cancer that is poorly responsive to current treatments. Minimally invasive, specific, and sensitive biomarkers providing early and effective diagnosis in high-risk patients are urgently needed. MicroRNAs (miRNAs, miRs) are endogenous, non-coding, small RNAs with established diagnostic value in cancer and pollution exposure. A systematic review and a qualitative meta-analysis were conducted to identify high-confidence miRNAs that can serve as biomarkers of asbestos exposure and MM.
Methods
The major biomedical databases were systematically searched for miRNA expression signatures related to asbestos exposure and MM. The qualitative meta-analysis applied a novel vote-counting method that takes into account multiple parameters. The most significant miRNAs thus identified were then subjected to functional and bioinformatic analysis to assess their biomarker potential.
Results
A pool of deregulated circulating and tissue miRNAs with biomarker potential for MM was identified and designated as “mesomiRs” (MM-associated miRNAs). Comparison of data from asbestos-exposed and MM subjects found that the most promising candidates for a multimarker signature were circulating miR-126-3p, miR-103a-3p, and miR-625-3p in combination with mesothelin. The most consistently described tissue miRNAs, miR-16-5p, miR-126-3p, miR-143-3p, miR-145-5p, miR-192-5p, miR-193a-3p, miR-200b-3p, miR-203a-3p, and miR-652-3p, were also found to provide a diagnostic signature and should be further investigated as possible therapeutic targets.
Conclusion
The qualitative meta-analysis and functional investigation confirmed the early diagnostic value of two miRNA signatures for MM. Large-scale, standardized validation studies are needed to assess their clinical relevance, so as to move from the workbench to the clinic.
REFERENCES
1.
IARC Arsenic, metals, fibres, and dusts. IARC Monogr Eval Carcinog Risks Hum. 2012;100:11–465. [PMC free article] [PubMed]2.
LaDou J. The asbestos cancer epidemic. Environ Health Perspect. 2004;112:285–90. [PMC free article] [PubMed]3.
Frank AL, Joshi TK. The global spread of asbestos. Ann Glob Heal. 2014;80:257–62. [PubMed]4.
Nishikawa K, Takahashi K, Karjalainen A, Wen C-P, Furuya S, Hoshuyama T, Todoroki M, Kiyomoto Y, Wilson D, Higashi T, Ohtaki M, Pan G, Wagner G. Recent mortality from pleural mesothelioma, historical patterns of asbestos use, and adoption of bans: a global assessment. Environ Health Perspect. 2008;116:1675–80. [PMC free article] [PubMed]5.
Mensi C, Riboldi L, De Matteis S, Bertazzi PA, Consonni D. Impact of an asbestos cement factory on mesothelioma incidence: global assessment of effects of occupational, familial, and environmental exposure. Environ Int. 2015;74:191–9. [PubMed]6.
Marinaccio A, Binazzi A, Bonafede M, Corfiati M, Di Marzio D, Scarselli A, Verardo M, Mirabelli D, Gennaro V, Mensi C, Schallemberg G, Merler E, Negro C, et al. Malignant mesothelioma due to non-occupational asbestos exposure from the Italian national surveillance system (ReNaM): epidemiology and public health issues. Occup Environ Med. 2015;72:648–55. [PubMed]7.
Gwinn MR, DeVoney D, Jarabek AM, Sonawane B, Wheeler J, Weissman DN, Masten S, Thompson C. Meeting report: mode(s) of action of asbestos and related mineral fibers. Environ Health Perspect. 2011;119:1806–10. [PMC free article] [PubMed]8.
Baumann F, Ambrosi JP, Carbone M. The Lancet. Oncology. 2013;14:576–578. [PubMed]9.
Carbone M, Baris YI, Bertino P, Brass B, Comertpay S, Dogan AU, Gaudino G, Jube S, Kanodia S, Partridge CR, Pass HI, Rivera ZS, Steele I, Tuncer M, Way S, Yang H, Miller A. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc Natl Acad Sci U S A. 2011;108:13618–13623. [PMC free article] [PubMed]10.
Benedetti S, Nuvoli B, Catalani S, Galati R. Reactive oxygen species a double-edged sword for mesothelioma. Oncotarget. 2015;6:16848–65. doi: 10.18632/oncotarget.4253. [PMC free article] [PubMed] [Cross Ref]11.
Yusa T, Hiroshima K, Sakai F, Kishimoto T, Ohnishi K, Usami I, Morikawa T, Wu D, Itoi K, Okamoto K, Shinohara Y, Kohyama N, Morinaga K. Significant relationship between the extent of pleural plaques and pulmonary asbestos body concentration in lung cancer patients with occupational asbestos exposure. Am J Ind Med. 2015;58:444–55. [PubMed]12.
Elshazley M, Shibata E, Hisanaga N, Ichihara G, Ewis AA, Kamijima M, Ichihara S, Sakai K, Sato M, Kondo M, Hasegawa Y. Pleural plaque profiles on the chest radiographs and CT scans of asbestos-exposed Japanese construction workers. Ind Health. 2011;49:626–33. [PubMed]13.
Napolitano A, Antoine DJ, Pellegrini L, Baumann F, Pagano IS, Pastorino S, Goparaju CM, Prokrym K, Canino C, Pass H, Carbone M, Yang H. HMGB1 and its hyper-acetylated isoform are sensitive and specific serum biomarkers to detect asbestos exposure and to identify mesothelioma patients. Clin Cancer Res. 2016 [PMC free article] [PubMed]14.
de Assis LVM, Locatelli J, Isoldi MC. The role of key genes and pathways involved in the tumorigenesis of Malignant Mesothelioma. Biochim Biophys Acta. 2014;1845:232–47. [PubMed]15.
Norbet C, Joseph A, Rossi SS, Bhalla S, Gutierrez FR. Asbestos-Related Lung Disease: A Pictorial Review. Curr Probl Diagn Radiol. 2015;44:371–82. [PubMed]16.
Consensus report. Asbestos, asbestosis, and cancer: The Helsinkicriteria for diagnosis and attribution. Scand J Work Environ Health. 1997;23:311–316. [PubMed]17.
Cullen MR, Barnett MJ, Balmes JR, Cartmel B, Redlich CA, Brodkin CA, Barnhart S, Rosenstock L, Goodman GE, Hammar SP, Thornquist MD, Omenn GS. Predictors of lung cancer among asbestos-exposed men in the {beta}-carotene and retinol efficacy trial. Am. J. Epidemiol. 2005;161:260–270. [PubMed]18.
Pass HI, Lott D, Lonardo F, Harbut M, Liu Z, Tang N, Carbone M, Webb C, Wali A. Asbestos Exposure, Pleural Mesothelioma, and Serum Osteopontin Levels. N Engl J Med. 2005;353:1564–73. [PubMed]19.
Delgermaa V, Takahashi K, Park EK, Le GV, Hara T, Sorahan T. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ. 2011;89:716–24. 724A-724C. [PMC free article] [PubMed]20.
Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr Treat Options Oncol. 2008;9:147–57. [PMC free article] [PubMed]21.
Ahmed I, Ahmed Tipu S, Ishtiaq S. Malignant mesothelioma. Pakistan J Med Sci. 2013;29:1433–8. [PMC free article] [PubMed]22.
Carbone M, Rizzo P, Pass H. Simian virus 40: the link with human malignant mesothelioma is well established. Anticancer Res. 2000;20:875–7. [PubMed]23.
Carbone M. Simian virus 40 and human tumors: It is time to study mechanisms. J Cell Biochem. 1999;76:189–93. [PubMed]24.
Gazdar AF, Carbone M. Molecular pathogenesis of malignant mesothelioma and its relationship to simian virus 40. Clin Lung Cancer. 2003;5:177–81. [PubMed]25.
Bocchetta M, Eliasz S, De Marco MA, Rudzinski J, Zhang L, Carbone M. The SV40 large T antigen-p53 complexes bind and activate the insulin-like growth factor-I promoter stimulating cell growth. Cancer Res. 2008;68:1022–9. [PubMed]26.
Carbone M, Pannuti A, Zhang L, Testa JR, Bocchetta M. A novel mechanism of late gene silencing drives SV40 transformation of human mesothelial cells. Cancer Res. 2008;68:9488–96. [PMC free article] [PubMed]27.
Stahel RA, Weder W, Lievens Y, Felip E. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v126–8. [PubMed]28.
Porpodis K, Zarogoulidis P, Boutsikou E, Papaioannou A, Machairiotis N, Tsakiridis K, Katsikogiannis N, Zaric B, Perin B, Huang H, Kougioumtzi I, Spyratos D, Zarogoulidis K. Malignant pleural mesothelioma: current and future perspectives. J Thorac Dis. 2013;5:S397–406. [PMC free article] [PubMed]29.
Bianchi C, Bianchi T. Global mesothelioma epidemic: Trend and features. Indian J Occup Environ Med. 2014;18:82–8. [PMC free article] [PubMed]30.
Hashim D, Boffetta P. Occupational and Environmental Exposures and Cancers in Developing Countries. Ann Glob Heal. 2014;80:393–411. [PubMed]31.
Pasetto R, Terracini B, Marsili D, Comba P. Occupational burden of asbestos-related cancer in Argentina, Brazil, Colombia, and Mexico. Ann Glob Heal. 2014;80:263–8. [PubMed]32.
Park E-K, Takahashi K, Hoshuyama T, Cheng T-J, Delgermaa V, Le GV, Sorahan T. Global magnitude of reported and unreported mesothelioma. Environ Health Perspect. 2011;119:514–8. [PMC free article] [PubMed]33.
Baumann F, Buck BJ, Metcalf R V, McLaurin BT, Merkler DJ, Carbone M. The Presence of Asbestos in the Natural Environment is Likely Related to Mesothelioma in Young Individuals and Women from Southern Nevada. J Thorac Oncol. 2015;10:731–7. [PMC free article] [PubMed]34.
Thomas A, Chen Y, Yu T, Gill A, Prasad V. Distinctive clinical characteristics of malignant mesothelioma in young patients. Oncotarget. 2015;6:16766–73. doi: 10.18632/oncotarget.4414. [PMC free article] [PubMed] [Cross Ref]35.
Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S, Hesdorffer M, Nasu M, Powers A, Rivera Z, Comertpay S, Tanji M, Gaudino G, Yang H, Carbone M. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–5. [PMC free article] [PubMed]36.
Carbone M, Flores EG, Emi M, Johnson TA, Tsunoda T, Behner D, Hoffman H, Hesdorffer M, Nasu M, Napolitano A, Powers A, Minaai M, Baumann F, Bryant-Greenwood P, Lauk O, Kirschner MB, Weder W, Opitz I, Pass HI, Gaudino G, Pastorino S, Yang H. Combined Genetic and Genealogic Studies Uncover a Large BAP1 Cancer Syndrome Kindred Tracing Back Nine Generations to a Common Ancestor from the 1700s. PLoS Genet. 2015;11:e1005633. [PMC free article] [PubMed]37.
Baumann F, Maurizot P, Mangeas M, Ambrosi JP, Douwes J, Robineau B. Pleural mesothelioma in New Caledonia: associations with environmental risk factors. Environ Health Perspect. 2011;119:695–700. [PMC free article] [PubMed]38.
Husain AN, Colby T, Ordonez N, Krausz T, Attanoos R, Beasley MB, Borczuk AC, Butnor K, Cagle PT, Chirieac LR, Churg A, Dacic S, Fraire A, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2013;137:647–67. [PubMed]39.
Ettinger DS, Akerley W, Borghaei H, Chang A, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AKP, Govindan R, Grannis FW, Horn L, Jahan TM, et al. Malignant Pleural Mesothelioma. J Natl Compr Canc Netw. 2012;10:26–41. [PubMed]40.
Rippo MR, Moretti S, Vescovi S, Tomasetti M, Orecchia S, Amici G, Catalano A, Procopio A. FLIP overexpression inhibits death receptor-induced apoptosis in malignant mesothelial cells. Oncogene. 2004;23:7753–60. [PubMed]41.
Henderson DW, Reid G, Kao SC, van Zandwijk N, Klebe S. Challenges and controversies in the diagnosis of malignant mesothelioma: Part 2. Malignant mesothelioma subtypes, pleural synovial sarcoma, molecular and prognostic aspects of mesothelioma, BAP1, aquaporin-1 and microRNA. J Clin Pathol. 2013;66:854–61. [PubMed]42.
Opitz I. Management of malignant pleural mesothelioma-The European experience. J Thorac Dis. 2014;6:S238–52. [PMC free article] [PubMed]43.
Greillier L, Baas P, Welch JJ, Hasan B, Passioukov A. Biomarkers for malignant pleural mesothelioma: current status. Mol Diagn Ther. 2008;12:375–90. [PubMed]44.
Hollevoet K, Reitsma JB, Creaney J, Grigoriu BD, Robinson BW, Scherpereel A, Cristaudo A, Pass HI, Nackaerts K, Rodríguez Portal JA, Schneider J, Muley T, Di Serio F, et al. Serum mesothelin for diagnosing malignant pleural mesothelioma: an individual patient data meta-analysis. J Clin Oncol. 2012;30:1541–9. [PMC free article] [PubMed]45.
Hu Z-D, Liu X-F, Liu X-C, Ding C-M, Hu C-J. Diagnostic accuracy of osteopontin for malignant pleural mesothelioma: A systematic review and meta-analysis. Clin Chim Acta. 2014;433:44–8. [PubMed]46.
Pantazopoulos I, Boura P, Xanthos T, Syrigos K. Effectiveness of mesothelin family proteins and osteopontin for malignant mesothelioma. Eur Respir J. 2013;41:706–15. [PubMed]47.
Panou V, Vyberg M, Weinreich UM, Meristoudis C, Falkmer UG, Røe OD. The established and future biomarkers of malignant pleural mesothelioma. Cancer Treat Rev. 2015;41:486–95. [PubMed]48.
Sheinerman KS, Umansky SR. Circulating cell-free microRNA as biomarkers for screening, diagnosis and monitoring of neurodegenerative diseases and other neurologic pathologies. Front Cell Neurosci. 2013;7:150. [PMC free article] [PubMed]49.
Carrington JC, Ambros V. Role of microRNAs in plant and animal development. Science. 2003;301:336–8. [PubMed]50.
Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–7. [PubMed]51.
Suárez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res. 2009;104:442–54. [PMC free article] [PubMed]52.
Olivieri F, Rippo MR, Prattichizzo F, Babini L, Graciotti L, Recchioni R, Procopio AD. Toll like receptor signaling in “inflammaging”: microRNA as new players. Immun Ageing. 2013;10:11. [PMC free article] [PubMed]53.
Olivieri F, Rippo MR, Procopio AD, Fazioli F. Circulating inflamma-miRs in aging and age-related diseases. Front Genet. 2013;4:121. [PMC free article] [PubMed]54.
Rippo MR, Olivieri F, Monsurrò V, Prattichizzo F, Albertini MC, Procopio AD. MitomiRs in human inflamm-aging: a hypothesis involving miR-181a, miR-34a and miR-146a. Exp Gerontol. 2014;56:154–63. [PubMed]55.
Cote GA, Gore AJ, McElyea SD, Heathers LE, Xu H, Sherman S, Korc M. A Pilot Study to Develop a Diagnostic Test for Pancreatic Ductal Adenocarcinoma Based on Differential Expression of Select miRNA in Plasma and Bile. Am J Gastroenterol. 2014;109:1942–52. [PMC free article] [PubMed]56.
Boisen MK, Dehlendorff C, Linnemann D, Nielsen BS, Larsen JS, Osterlind K, Nielsen SE, Tarpgaard LS, Qvortrup C, Pfeiffer P, Holländer NH, Keldsen N, Hansen TF, et al. Tissue MicroRNAs as Predictors of Outcome in Patients with Metastatic Colorectal Cancer Treated with First Line Capecitabine and Oxaliplatin with or without Bevacizumab. PLoS One. 2014;9:e109430. [PMC free article] [PubMed]57.
Fortunato O, Boeri M, Verri C, Conte D, Mensah M, Suatoni P, Pastorino U, Sozzi G. Assessment of circulating microRNAs in plasma of lung cancer patients. Molecules. 2014;19:3038–54. [PubMed]58.
Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–8. [PubMed]59.
Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med. 2009;60:167–79. [PubMed]60.
Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002;99:15524–9. [PMC free article] [PubMed]61.
Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101:2999–3004. [PMC free article] [PubMed]62.
Sevignani C, Calin GA, Nnadi SC, Shimizu M, Davuluri R V, Hyslop T, Demant P, Croce CM, Siracusa LD. MicroRNA genes are frequently located near mouse cancer susceptibility loci. Proc Natl Acad Sci USA. 2007;104:8017–22. [PMC free article] [PubMed]63.
Sozzi G, Boeri M, Rossi M, Verri C, Suatoni P, Bravi F, Roz L, Conte D, Grassi M, Sverzellati N, Marchiano A, Negri E, La Vecchia C, Pastorino U. Clinical utility of a plasma-based miRNA signature classifier within computed tomography lung cancer screening: a correlative MILD trial study. J Clin Oncol. 2014;32:768–73. [PMC free article] [PubMed]64.
Vrijens K, Bollati V, Nawrot TS. MicroRNAs as Potential Signatures of Environmental Exposure or Effect: A Systematic Review. Environ Health Perspect. 2015;123:399–411. [PMC free article] [PubMed]65.
Lo Cicero A, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 2015;35:69–77. [PubMed]66.
Zhao L, Liu W, Xiao J, Cao B. The role of exosomes and “exosomal shuttle microRNA” in tumorigenesis and drug resistance. Cancer Lett. 2015;356:339–46. [PubMed]67.
Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–33. [PMC free article] [PubMed]68.
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–8. [PMC free article] [PubMed]69.
Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–33. [PMC free article] [PubMed]70.
Nymark P, Guled M, Borze I, Faisal A, Lahti L, Salmenkivi K, Kettunen E, Anttila S, Knuutila S. Integrative analysis of microRNA, mRNA and aCGH data reveals asbestos- and histology-related changes in lung cancer. Genes Chromosomes Cancer. 2011;50:585–97. [PubMed]71.
Guled M, Lahti L, Lindholm PM, Salmenkivi K, Bagwan I. CDKN2A, NF2, and JUN Are Dysregulated Among Other Genes by miRNAs in Malignant Mesothelioma — A miRNA Microarray Analysis. Genes Chromosomes Cancer. 2009;48:615–23. [PubMed]72.
Gee G V, Koestler DC, Christensen BC, Sugarbaker DJ, Ugolini D, Ivaldi GP, Resnick MB, Houseman EA, Kelsey KT, Marsit CJ. Downregulated microRNAs in the differential diagnosis of malignant pleural mesothelioma. Int J Cancer. 2010;127:2859–69. [PMC free article] [PubMed]73.
Benjamin H, Lebanony D, Rosenwald S, Cohen L, Gibori H, Barabash N, Ashkenazi K, Goren E, Meiri E, Morgenstern S, Perelman M, Barshack I, Goren Y, et al. A diagnostic assay based on microRNA expression accurately identifies malignant pleural mesothelioma. J Mol Diagn. 2010;12:771–9. [PMC free article] [PubMed]74.
Pass HI, Goparaju C, Ivanov S, Donington J, Carbone M, Hoshen M, Cohen D, Chajut A, Rosenwald S, Dan H, Benjamin S, Aharonov R. hsa-miR-29c* is linked to the prognosis of malignant pleural mesothelioma. Cancer Res. 2010;70:1916–24. [PMC free article] [PubMed]75.
Xu Y, Zheng M, Merritt RE, Shrager JB, Wakelee H, Kratzke RA, Hoang CD. miR-1 induces growth arrest and apoptosis in malignant mesothelioma. Chest. 2013;144:1632–43. [PMC free article] [PubMed]76.
Cioce M, Ganci F, Canu V, Sacconi A, Mori F, Canino C, Korita E, Casini B, Alessandrini G, Cambria A, Carosi MA, Blandino R, Panebianco V, et al. Protumorigenic effects of mir-145 loss in malignant pleural mesothelioma. Oncogene. 2013;33:5319–31. [PMC free article] [PubMed]77.
Andersen M, Grauslund M, Ravn J, Sørensen JB, Andersen CB, Santoni-Rugiu E. Diagnostic Potential of miR-126, miR-143, miR-145, and miR-652 in Malignant Pleural Mesothelioma. J Mol Diagn. 2014;16:418–30. [PubMed]78.
Ramírez-Salazar EG, Salinas-Silva LC, Vázquez-Manríquez ME, Gayosso-Gómez LV, Negrete-Garcia MC, Ramírez-Rodriguez SL, Chávez R, Zenteno E, Santillán P, Kelly-García J, Ortiz-Quintero B. Analysis of microRNA expression signatures in malignant pleural mesothelioma, pleural inflammation, and atypical mesothelial hyperplasia reveals common predictive tumorigenesis-related targets. Exp Mol Pathol. 2014;97:375–85. [PubMed]79.
Kirschner MB, Cheng YY, Armstrong NJ, Lin RCY, Kao SC, Linton A, Klebe S, McCaughan BC, van Zandwijk N, Reid G. MiR-Score: A novel 6-microRNA signature that predicts survival outcomes in patients with malignant pleural mesothelioma. Mol Oncol. 2015;9:715–26. [PubMed]80.
Ak G, Tomaszek SC, Kosari F, Metintas M, Jett JR, Metintas S, Yildirim H, Dundar E, Dong J, Aubry MC, Wigle DA, Thomas CF. MicroRNA and mRNA features of malignant pleural mesothelioma and benign asbestos-related pleural effusion. Biomed Res Int. 2015;2015:635748. [PMC free article] [PubMed]81.
Balatti V, Maniero S, Ferracin M, Veronese A. MicroRNAs Dysregulation in Human Malignant Pleural Mesothelioma. 2011;6:844–51. [PubMed]82.
Busacca S, Germano S, De Cecco L, Rinaldi M, Comoglio F, Favero F, Murer B, Mutti L, Pierotti M, Gaudino G. MicroRNA signature of malignant mesothelioma with potential diagnostic and prognostic implications. Am J Respir Cell Mol Biol. 2010;42:312–9. [PubMed]83.
Andersen M, Grauslund M, Muhammad-Ali M, Ravn J, Sørensen JB, Andersen CB, Santoni-Rugiu E. Are differentially expressed microRNAs useful in the diagnostics of malignant pleural mesothelioma? APMIS. 2012;120:767–9. [PubMed]84.
Ivanov S V, Goparaju CM V, Lopez P, Zavadil J, Toren-Haritan G, Rosenwald S, Hoshen M, Chajut A, Cohen D, Pass HI. Pro-tumorigenic effects of miR-31 loss in mesothelioma. J Biol Chem. 2010;285:22809–17. [PMC free article] [PubMed]85.
Santarelli L, Strafella E, Staffolani S, Amati M, Emanuelli M, Sartini D, Pozzi V, Carbonari D, Bracci M, Pignotti E, Mazzanti P, Sabbatini A, Ranaldi R, et al. Association of MiR-126 with soluble mesothelin-related peptides, a marker for malignant mesothelioma. PLoS One. 2011;6:e18232. [PMC free article] [PubMed]86.
Tomasetti M, Staffolani S, Nocchi L, Neuzil J, Strafella E, Manzella N, Mariotti L, Bracci M, Valentino M, Amati M, Santarelli L. Clinical significance of circulating miR-126 quantification in malignant mesothelioma patients. Clin Biochem. 2012;45:575–81. [PubMed]87.
Häusler SFM, Keller A, Chandran PA, Ziegler K, Zipp K, Heuer S, Krockenberger M, Engel JB, Hönig A, Scheffler M, Dietl J, Wischhusen J. Whole blood-derived miRNA profiles as potential new tools for ovarian cancer screening. Br J Cancer. 2010;103:693–700. [PMC free article] [PubMed]88.
Weber DG, Johnen G, Bryk O, Jöckel K-H, Brüning T. Identification of miRNA-103 in the cellular fraction of human peripheral blood as a potential biomarker for malignant mesothelioma--a pilot study. PLoS One. 2012;7:e30221. [PMC free article] [PubMed]89.
Weber DG, Casjens S, Johnen G, Bryk O, Raiko I, Pesch B, Kollmeier J, Bauer TT, Brüning T. Combination of MiR-103a-3p and mesothelin improves the biomarker performance of malignant mesothelioma diagnosis. PLoS One. 2014;9:e114483. [PMC free article] [PubMed]90.
Kirschner MB, Cheng YY, Badrian B, Kao SC, Creaney J, Edelman JJB, Armstrong NJ, Vallely MP, Musk AW, Robinson BWS, McCaughan BC, Klebe S, Mutsaers SE, et al. Increased circulating miR-625-3p: a potential biomarker for patients with malignant pleural mesothelioma. J Thorac Oncol. 2012;7:1184–91. [PubMed]91.
Lamberti M, Capasso R, Lombardi A, Di Domenico M, Fiorelli A, Feola A, Perna AF, Santini M, Caraglia M, Ingrosso D. Two Different Serum MiRNA Signatures Correlate with the Clinical Outcome and Histological Subtype in Pleural Malignant Mesothelioma Patients. PLoS One. 2015;10:e0135331. [PMC free article] [PubMed]92.
Fassina A, Cappellesso R, Guzzardo V, Dalla Via L, Piccolo S, Ventura L, Fassan M. Epithelial-mesenchymal transition in malignant mesothelioma. Mod Pathol. 2012;25:86–99. [PubMed]93.
Kemp CD, Rao M, Xi S, Inchauste S, Mani H, Fetsch P, Filie A, Zhang M, Hong J a, Walker RL, Zhu YJ, Ripley RT, Mathur A, et al. Polycomb repressor complex-2 is a novel target for mesothelioma therapy. Clin Cancer Res. 2012;18:77–90. [PubMed]94.
Reid G, Pel ME, Kirschner MB, Cheng YY, Mugridge N, Weiss J, Williams M, Wright C, Edelman JJB, Vallely MP, McCaughan BC, Klebe S, Brahmbhatt H, et al. Restoring expression of miR-16: a novel approach to therapy for malignant pleural mesothelioma. Ann Oncol. 2013;24:3128–35. [PubMed]95.
Cheng YY, Kirschner MB, Cheng NC, Gattani S, Klebe S, Edelman JJB, Vallely MP, McCaughan BC, Jin HC, van Zandwijk N, Reid G. ZIC1 is silenced and has tumor suppressor function in malignant pleural mesothelioma. J Thorac Oncol. 2013;8:1317–28. [PubMed]96.
Riquelme E, Suraokar MB, Rodriguez J, Mino B, Lin HY, Rice DC, Tsao A, Wistuba II. Frequent Coamplification and Cooperation between C-MYC and PVT1 Oncogenes Promote Malignant Pleural Mesothelioma. J Thorac Oncol. 2014;9:998–1007. [PMC free article] [PubMed]97.
Matsumoto S, Nabeshima K, Hamasaki M, Shibuta T, Umemura T. Upregulation of microRNA-31 associates with a poor prognosis of malignant pleural mesothelioma with sarcomatoid component. Med Oncol. 2014;31:303. [PubMed]98.
Birnie KA, Yip YY, Ng DCH, Kirschner MB, Reid G, Prele CM, Musk AWB, Lee YG, Thompson PJ, Mutsaers SE, Badrian B. Loss of mir-223 and JNK Signalling Contribute to Elevated Stathmin in Malignant Pleural Mesothelioma. Mol Cancer Res. 2015;13:1106–18. [PubMed]99.
Williams M, Kirschner MB, Cheng YY, Hanh J, Weiss J, Mugridge N, Wright CM, Linton A, Kao SC, Edelman JJB, Vallely MP, McCaughan BC, Cooper W, et al. miR-193a-3p is a potential tumor suppressor in malignant pleural mesothelioma. Oncotarget. 2015;6:23480–95. doi: 10.18632/oncotarget.4346. [PMC free article] [PubMed] [Cross Ref]100.
Crispi S, Cardillo I, Spugnini EP, Citro G, Menegozzo S, Baldi A. Biological agents involved in malignant mesothelioma: relevance as biomarkers or therapeutic targets. Curr Cancer Drug Targets. 2010;10:19–26. [PubMed]101.
Kubo T, Toyooka S, Tsukuda K, Sakaguchi M, Fukazawa T, Soh J, Asano H, Ueno T, Muraoka T, Yamamoto H, Nasu Y, Kishimoto T, Pass HI, et al. Epigenetic silencing of microRNA-34b/c plays an important role in the pathogenesis of malignant pleural mesothelioma. Clin Cancer Res. 2011;17:4965–74. [PubMed]102.
Tanaka N, Toyooka S, Soh J, Tsukuda K, Shien K, Furukawa M, Muraoka T, Maki Y, Ueno T, Yamamoto H, Asano H, Otsuki T, Miyoshi S. Downregulation of microRNA-34 induces cell proliferation and invasion of human mesothelial cells. Oncol Rep. 2013;29:2169–74. [PubMed]103.
Ueno T, Toyooka S, Fukazawa T, Kubo T, Soh J, Asano H, Muraoka T, Tanaka N, Maki Y, Shien K, Furukawa M, Sakaguchi M, Yamamoto H, et al. Preclinical evaluation of microRNA-34b/c delivery for malignant pleural mesothelioma. Acta Med Okayama. 2014;68:23–6. [PubMed]104.
Maki Y, Asano H, Toyooka S, Soh J, Kubo T, Katsui K, Ueno T, Shien K, Muraoka T, Tanaka N, Yamamoto H, Tsukuda K, Kishimoto T, et al. MicroRNA miR-34b/c enhances cellular radiosensitivity of malignant pleural mesothelioma cells. Anticancer Res. 2012;32:4871–5. [PubMed]105.
Ghawanmeh T, Thunberg U, Castro J, Murray F, Laytragoon-Lewin N. miR-34a expression, cell cycle arrest and cell death of malignant mesothelioma cells upon treatment with radiation, docetaxel or combination treatment. Oncology. 2011;81:330–5. [PubMed]106.
Muraoka T, Soh J, Toyooka S, Aoe K, Fujimoto N, Hashida S, Maki Y, Tanaka N, Shien K, Furukawa M, Yamamoto H, Asano H, Tsukuda K, et al. The degree of microRNA-34b/c methylation in serum-circulating DNA is associated with malignant pleural mesothelioma. Lung Cancer. 2013;82:485–90. [PubMed]107.
Goparaju CM, Blasberg JD, Volinia S, Palatini J, Ivanov S, Donington JS, Croce C, Carbone M, Yang H, Pass HI. Onconase mediated NFK downregulation in malignant pleural mesothelioma. Oncogene. 2011;30:2767–77. [PMC free article] [PubMed]108.
Nasu M, Carbone M, Gaudino G, Ly BH, Bertino P, Shimizu D, Morris P, Pass HI, Yang H. Ranpirnase interferes with NF-B Pathway and MMP9 activity, inhibiting Malignant Mesothelioma cell invasiveness and xenograft growth. Genes Cancer. 2011;2:576–584. doi: 10.1177/1947601911412375. [PMC free article] [PubMed] [Cross Ref]109.
Khodayari N, Mohammed KA, Goldberg EP, Nasreen N. EphrinA1 inhibits malignant mesothelioma tumor growth via let-7 microRNA-mediated repression of the RAS oncogene. Cancer Gene Ther. 2011;18:806–16. [PubMed]110.
Tomasetti M, Nocchi L, Staffolani S, Manzella N, Amati M, Goodwin J, Kluckova K, Nguyen M, Strafella E, Bajzikova M, Peterka M, Lettlova S, Truksa J, et al. MicroRNA-126 Suppresses Mesothelioma Malignancy by Targeting IRS1 and Interfering with the Mitochondrial Function. Antioxid Redox Signal. 2014;21:2109–25. [PMC free article] [PubMed]111.
Yamamoto K, Seike M, Takeuchi S, Soeno C, Miyanaga A, Noro R, Minegishi Y, Kubota K, Gemma A. miR-379/411 cluster regulates IL-18 and contributes to drug resistance in malignant pleural mesothelioma. Oncol Rep. 2014;32:2365–72. [PubMed]112.
Carbone M, Pass HI, Rizzo P, Marinetti M, Di Muzio M, Mew DJ, Levine AS, Procopio A. Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene. 1994;9:1781–90. [PubMed]113.
Carbone M, Rizzo P, Grimley PM, Procopio A, Mew DJ, Shridhar V, de Bartolomeis A, Esposito V, Giuliano MT, Steinberg SM, Levine AS, Giordano A, Pass HI. Simian virus-40 large-T antigen binds p53 in human mesotheliomas. Nat Med. 1997;3:908–12. [PubMed]114.
Bocchetta M, Carbone M. SV40 Tag/p53 complexes actively promote malignant cell growth of human mesothelial cells. Cell Oncol. 2008;30:455. [PMC free article] [PubMed]115.
Bocchetta M, Eliasz S, De Marco MA, Rudzinski J, Zhang L, Carbone M. The SV40 large T antigen-p53 complexes bind and activate the insulin-like growth factor-I promoter stimulating cell growth. Cancer Res. 2008;68:1022–9. [PubMed]116.
Sullivan CS, Grundhoff AT, Tevethia S, Pipas JM, Ganem D. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature. 2005;435:682–6. [PubMed]117.
Sullivan CS, Grundhoff AT, Tevethia S, Pipas JM, Ganem D. SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature. 2005;435:682–6. [PubMed]118.
Chen CJ, Burke JM, Kincaid RP, Azarm KD, Mireles N, Butel JS, Sullivan CS. Naturally arising strains of polyomaviruses with severely attenuated microRNA expression. J Virol. 2014;88:12683–93. [PMC free article] [PubMed]119.
Sevinc ED, Egeli U, Cecener G, Tezcan G, Tunca B, Gokgoz S, Tasdelen I, Tolunay S, Evrensel T. Association of miR-1266 with recurrence/metastasis potential in estrogen receptor positive breast cancer patients. Pac J Cancer Prev. 2015;16:291–7. [PubMed]120.
Chen L, Lü MH, Zhang D, Hao NB, Fan YH, Wu YY, Wang SM, Xie R, Fang DC, Zhang H, Hu CJ, Yang SM. miR-1207-5p and miR-1266 suppress gastric cancer growth and invasion by targeting telomerase reverse transcriptase. Cell Death Dis. 2014;5:e1034. [PMC free article] [PubMed]121.
Ichihara A, Jinnin M, Oyama R, Yamane K, Fujisawa A, Sakai K, Masuguchi S, Fukushima S, Maruo K, Ihn H. Increased serum levels of miR-1266 in patients with psoriasis vulgaris. Eur J Dermatol. 2012;22:68–71. [PubMed]122.
Gee GV, Stanifer ML, Christensen BC, Atwood WJ, Ugolini D, Bonassi S, Resnick MB, Nelson HH, Marsit CJ, Kelsey KT. SV40 associated miRNAs are not detectable in mesotheliomas. Br J Cancer. 2010;103:885–8. [PMC free article] [PubMed]123.
Testa JR, Carbone M, Hirvonen A, Khalili K, Krynska B, Linnainmaa K, Pooley FD, Rizzo P, Rusch V, Xiao GH. A multi-institutional study confirms the presence and expression of simian virus 40 in human malignant mesotheliomas. Cancer Res. 1998;58:4505–9. [PubMed]124.
Hirvonen A, Mattson K, Karjalainen A, Ollikainen T, Tammilehto L, Hovi T, Vainio H, Pass HI, Di Resta I, Carbone M, Linnainmaa K. Simian virus 40 (SV40)-like DNA sequences not detectable in finnish mesothelioma patients not exposed to SV40-contaminated polio vaccines. Mol Carcinog. 1999;26:93–9. [PubMed]125.
Carbone M, Fisher S, Powers A, Pass HI, Rizzo P. New molecular and epidemiological issues in mesothelioma: role of SV40. J Cell Physiol. 1999;180:167–72. [PubMed]126.
Xue Z, Wen J, Chu X, Xue X. A microRNA gene signature for identification of lung cancer. Surg Oncol. 2014;23:126–31. [PubMed]127.
Del Vescovo V, Grasso M, Barbareschi M, Denti MA. MicroRNAs as lung cancer biomarkers. World J Clin Oncol. 2014;5:604–20. [PMC free article] [PubMed]128.
Lan H, Lu H, Wang X, Jin H. MicroRNAs as Potential Biomarkers in Cancer: Opportunities and Challenges. Biomed Res Int. 2015;2015:125094. [PMC free article] [PubMed]129.
Reid G, Kirschner MB, van Zandwijk N. Circulating microRNAs: Association with disease and potential use as biomarkers. Crit Rev Oncol Hematol. 2011;80:193–208. [PubMed]130.
Russo F, Di Bella S, Bonnici V, Laganà A, Rainaldi G, Pellegrini M, Pulvirenti A, Giugno R, Ferro A. A knowledge base for the discovery of function, diagnostic potential and drug effects on cellular and extracellular miRNAs. BMC Genomics. 2014;15(Suppl 3):S4. [PMC free article] [PubMed]131.
Russo F, Di Bella S, Nigita G, Macca V, Laganà A, Giugno R, Pulvirenti A, Ferro A. miRandola: extracellular circulating microRNAs database. PLoS One. 2012;7:e47786. [PMC free article] [PubMed]132.
Gandellini P, Profumo V, Folini M, Zaffaroni N. MicroRNAs as new therapeutic targets and tools in cancer. Expert Opin Ther Target. 2011;15:265–79. [PubMed]133.
Suzuki H, Maruyama R, Yamamoto E, Kai M. Epigenetic alteration and microRNA dysregulation in cancer. Front Genet. 2013;4:258. [PMC free article] [PubMed]134.
He L, He X, Lowe SW, Hannon GJ. microRNAs join the p53 network--another piece in the tumour-suppression puzzle. Nat Rev Cancer. 2007;7:819–22. [PMC free article] [PubMed]135.
John-Aryankalayil M, Palayoor ST, Makinde AY, Cerna D, Simone CB, Falduto MT, Magnuson SR, Coleman CN. Fractionated radiation alters oncomir and tumor suppressor miRNAs in human prostate cancer cells. Radiat Res. 2012;178:105–17. [PMC free article] [PubMed]136.
Liu C, Yu J, Yu S, Lavker RM, Cai L, Liu W, Yang K, He X, Chen S. MicroRNA-21 acts as an oncomir through multiple targets in human hepatocellular carcinoma. J Hepatol. 2010;53:98–107. [PubMed]137.
Takeda M, Kasai T, Enomoto Y, Takano M, Morita K, Kadota E, Iizuka N, Maruyama H, Nonomura A. Genomic gains and losses in malignant mesothelioma demonstrated by FISH analysis of paraffin-embedded tissues. J Clin Pathol. 2012;65:77–82. [PubMed]138.
Guo G, Chmielecki J, Goparaju C, Heguy A, Dolgalev I, Carbone M, Seepo S, Meyerson M, Pass HI. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res. 2015;75:264–9. [PubMed]139.
Neragi-Miandoab S, Sugarbaker DJ. Chromosomal deletion in patients with malignant pleural mesothelioma. Interact Cardiovasc Thorac Surg. 2009;9:42–4. [PubMed]140.
Serio G, Gentile M, Pennella A, Marzullo A, Buonadonna AL, Nazzaro P, Testini M, Musti M, Scattone A. Characterization of a complex chromosome aberration in two cases of peritoneal mesothelioma arising primarily in the hernial sac. Pathol Int. 2009;59:415–21. [PubMed]141.
Simon F, Johnen G, Krismann M, Müller K-M. Chromosomal alterations in early stages of malignant mesotheliomas. Virchows Arch. 2005;447:762–7. [PubMed]142.
Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, Taccioli C, Zanesi N, Garzon R, Aqeilan RI, Alder H, Volinia S, Rassenti L, et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci USA. 2008;105:5166–71. [PMC free article] [PubMed]143.
Fish JE, Santoro MM, Morton SU, Yu S, Yeh R-F, Wythe JD, Ivey KN, Bruneau BG, Stainier DYR, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–84. [PMC free article] [PubMed]144.
Shen J, Xia W, Khotskaya YB, Huo L, Nakanishi K, Lim S-O, Du Y, Wang Y, Chang W-C, Chen C-H, Hsu JL, Wu Y, Lam YC, et al. EGFR modulates microRNA maturation in response to hypoxia through phosphorylation of AGO2. Nature. 2013;497:383–7. [PMC free article] [PubMed]145.
Kao SC, Fulham M, Wong K, Cooper W, Brahmbhatt H, MacDiarmid J, Pattison S, Sagong JO, Huynh Y, Leslie F, Pavlakis N, Clarke S, Boyer M, et al. A Significant Metabolic and Radiological Response after a Novel Targeted MicroRNA-based Treatment Approach in Malignant Pleural Mesothelioma. Am J Respir Crit Care Med. 2015;191:1467–9. [PubMed]146.
Tanaka N, Toyooka S, Soh J, Kubo T, Yamamoto H, Maki Y, Muraoka T, Shien K, Furukawa M, Ueno T, Asano H, Tsukuda K, Aoe K, Miyoshi S. Frequent methylation and oncogenic role of microRNA-34b/c in small-cell lung cancer. Lung Cancer. 2012;76:32–8. [PubMed]147.
Comertpay S, Pastorino S, Tanji M, Mezzapelle R, Strianese O, Napolitano A, Baumann F, Weigel T, Friedberg J, Sugarbaker P, Krausz T, Wang E, Powers A, et al. Evaluation of clonal origin of malignant mesothelioma. J Transl Med. 2014;12:301. [PMC free article] [PubMed]148.
de Giorgio A, Castellano L, Krell J, Stebbing J. Crosstalk-induced loss of miR-126 promotes angiogenesis. Oncogene. 2013;33:3634–5. [PubMed]149.
Ebrahimi F, Gopalan V, Smith RA, Lam AK-Y. miR-126 in human cancers: clinical roles and current perspectives. Exp Mol Pathol. 2014;96:98–107. [PubMed]150.
Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM, O'Connell RM. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321. [PMC free article] [PubMed]151.
Warnecke-Eberz U, Chon S-H, Hölscher AH, Drebber U, Bollschweiler E. Exosomal onco-miRs from serum of patients with adenocarcinoma of the esophagus: comparison of miRNA profiles of exosomes and matching tumor. Tumour Biol. 2015;36:4643–53. [PubMed]152.
Liu B, Peng X-C, Zheng X-L, Wang J, Qin Y-W. MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer. 2009;66:169–75. [PubMed]153.
Catalano A, Romano M, Martinotti S, Procopio A. Enhanced expression of vascular endothelial growth factor (VEGF) plays a critical role in the tumor progression potential induced by simian virus 40 large T antigen. Oncogene. 2002;21:2896–900. [PubMed]154.
Li Z, Li N, Wu M, Li X, Luo Z, Wang X. Expression of miR-126 suppresses migration and invasion of colon cancer cells by targeting CXCR4. Mol Cell Biochem. 2013;381:233–42. [PubMed]155.
Zhang Y, Yang P, Sun T, Li D, Xu X, Rui Y, Li C, Chong M, Ibrahim T, Mercatali L, Amadori D, Lu X, Xie D, Li Q-J, Wang X-F. miR-126 and miR-126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasis. Nat Cell Biol. 2013;15:284–94. [PMC free article] [PubMed]156.
Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MÁ, Bernad A, Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. [PMC free article] [PubMed]157.
Matsuzaki H, Maeda M, Lee S, Nishimura Y, Kumagai-Takei N, Hayashi H, Yamamoto S, Hatayama T, Kojima Y, Tabata R, Kishimoto T, Hiratsuka J, Otsuki T. Asbestos-induced cellular and molecular alteration of immunocompetent cells and their relationship with chronic inflammation and carcinogenesis. J Biomed Biotechnol. 2012;2012:492608. [PMC free article] [PubMed]158.
Kumagai-Takei N, Nishimura Y, Maeda M, Hayashi H, Matsuzaki H, Lee S, Hiratsuka J, Otsuki T. Effect of asbestos exposure on differentiation of cytotoxic T lymphocytes in mixed lymphocyte reaction of human peripheral blood mononuclear cells. Am J Respir Cell Mol Biol. American Thoracic Society. 2013;49:28–36. [PubMed]159.
Kumagai-Takei N, Nishimura Y, Maeda M, Hayashi H, Matsuzaki H, Lee S, Kishimoto T, Fukuoka K, Nakano T, Otsuki T. Functional properties of CD8(+) lymphocytes in patients with pleural plaque and malignant mesothelioma. J Immunol Res. 2014;2014:670140. [PMC free article] [PubMed]160.
Yang Y-L, Xu L-P, Zhuo F-L, Wang T-Y. Prognostic value of microRNA-10b overexpression in peripheral blood mononuclear cells of nonsmall-cell lung cancer patients. Tumour Biol. 2015;36:7069–75. [PubMed]161.
Roth C, Stückrath I, Pantel K, Izbicki JR, Tachezy M, Schwarzenbach H. Low levels of cell-free circulating miR-361-3p and miR-625* as blood-based markers for discriminating malignant from benign lung tumors. PLoS One. 2012;7:e38248. [PMC free article] [PubMed]162.
Duttagupta R, Jiang R, Gollub J, Getts RC, Jones KW. Impact of cellular miRNAs on circulating miRNA biomarker signatures. PLoS One. 2011;6:e20769. [PMC free article] [PubMed]163.
Ashby J, Flack K, Jimenez LA, Duan Y, Khatib A-K, Somlo G, Wang SE, Cui X, Zhong W. Distribution profiling of circulating microRNAs in serum. Anal Chem. 2014;86:9343–9. [PubMed]164.
Cui A, Jin X-G, Zhai K, Tong Z-H, Shi H-Z. Diagnostic values of soluble mesothelin-related peptides for malignant pleural mesothelioma: updated meta-analysis. BMJ Open. 2014;4:e004145. [PMC free article] [PubMed]165.
Cuk K, Zucknick M, Heil J, Madhavan D, Schott S, Turchinovich A, Arlt D, Rath M, Sohn C, Benner A, Junkermann H, Schneeweiss A, Burwinkel B. Circulating microRNAs in plasma as early detection markers for breast cancer. Int J Cancer. 2013;132:1602–12. [PubMed]166.
Pritchard CC, Kroh E, Wood B, Arroyo JD, Dougherty KJ, Miyaji MM, Tait JF, Tewari M. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res. 2012;5:492–7. [PMC free article] [PubMed]167.
Kirschner MB, Edelman JJB, Kao SC-H, Vallely MP, van Zandwijk N, Reid G. The Impact of Hemolysis on Cell-Free microRNA Biomarkers. Front Genet. 2013;4:94. [PMC free article] [PubMed]168.
Sen D. Working with asbestos and the possible health risks. Occup Med (Lond) 2015;65:6–14. [PubMed]169.
Rikke BA, Wynes MW, Rozeboom LM, Barón AE, Hirsch FR. Independent validation test of the vote-counting strategy used to rank biomarkers from published studies. Biomark Med. 2015;9:751–61. [PMC free article] [PubMed]170.
Terra-Filho M, Bagatin E, Nery LE, Nápolis LM, Neder JA, Meirelles Gde S, Silva CI, Muller NL. Screening of miners and millers at decreasing levels of asbestos exposure: comparison of chest radiography and thin-section computed tomography. PLoS One. 2015;10:e0118585. [PMC free article] [PubMed]171.
Mastrangelo G, Ballarin MN, Bellini E, Bizzotto R, Zannol F, Gioffrè F, Gobbi M, Tessadri G, Marchiori L, Marangi G, Bozzolan S, Lange JH, Valentini F, Spolaore P. Feasibility of a screening programme for lung cancer in former asbestos workers. Occup Med (Lond) 2008;58:175–180. [PubMed]172.
Magnani C, Agudo A, González CA, Andrion A, Calleja A, Chellini E, Dalmasso P, Escolar A, Hernandez S, Ivaldi C, Mirabelli D, Ramirez J, Turuguet D, Usel M, Terracini B. Multicentric study on malignant pleural mesothelioma and non-occupational exposure to asbestos. Br J Cancer. 2000;83:104–11. [PMC free article] [PubMed]173.
Edgar R. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–10. [PMC free article] [PubMed]174.
Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, et al. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41:D991–5. [PMC free article] [PubMed]175.
Rustici G, Kolesnikov N, Brandizi M, Burdett T, Dylag M, Emam I, Farne A, Hastings E, Ison J, Keays M, Kurbatova N, Malone J, Mani R, et al. ArrayExpress update--trends in database growth and links to data analysis tools. Nucleic Acids Res. 2013;41:D987–90. [PMC free article] [PubMed]177.
Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–4. [PMC free article] [PubMed]178.
Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–8. [PMC free article] [PubMed]179.
Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–7. [PMC free article] [PubMed]180.
Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–73. [PMC free article] [PubMed]181.
Van Peer G, Lefever S, Anckaert J, Beckers A, Rihani A, Van Goethem A, Volders P-J, Zeka F, Ongenaert M, Mestdagh P, Vandesompele J. miRBase Tracker: keeping track of microRNA annotation changes. Database (Oxford) 2014 2014. pii: bau080. [PMC free article] [PubMed]182.
Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. [PMC free article] [PubMed]183.
Devillé Walter L, Buntinx Frank, Bouter Lex M, Montori Victor M, de Vet Henrica CW, van der Windt Danielle AWM, Bezemer P Dick. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. [PMC free article] [PubMed]184.
Higgins JP1, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [PMC free article] [PubMed]185.
Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 2014;15:293. [PMC free article] [PubMed]
Articles from Oncotarget are provided here courtesy of Impact Journals, LLC
longkanker, ranpirnase, mesothelioma, onconase
Gerelateerde artikelen




 1,2
1,2
Plaats een reactie ...
Reageer op "Mesothelioma - asbestkanker: Ranpirnase oftewel Onconase geeft een significant verschil in overleving bij mesothelioma"