Helpt u ons aan 500 donateurs om kanker-actueel online te kunnen houden?
21 november 2022: zie ook dit artikel: https://kanker-actueel.nl/NL/prostaatkankerpatienten-waarbij-dna-is-getest-via-in-bloed-circulerend-tumorweefsel-ctdna-hebben-bij-positieve-brca-1-en-2-en-atm-mutaties-meer-baat-bij-olaparib-in-vergelijking-met-enzalutamide-en-abiraterone.html
25 mei 2020: Lees ook dit artikel:
4 april 2018: lees ook dit artikel:
9 november 2015: Bron: N Engl J Med 2015; 373:1697-1708. DOI: 10.1056/NEJMoa1506859
Olaparib - (Lynparza) geeft alsnog een hoge response bij zwaar voorbehandelde mannen met hormoonresistente en uitbehandelde veruitgezaaide prostaatkanker. En bij wie de olaparib aansloeg - gemiddeld 33% (20 tot 48%) reageerde goed op dit medicijn - werd de mediane overlevingstijd zo goed als verdubbeld van 7,5 naar 13,8 maanden.
Opvallend is dat alle responders, dus de mannen waarbij de olaparib aansloeg, een of meerdere specifieke DNA mutaties hadden in de genen verantwoordelijk voor DNA herstel. Waaronder ook BRCA 1 en 2, die veel voorkomen bij eierstokkanker en borstkanker, waar olaparib ook goede resultaten geeft overigens. Dit alles blijkt uit een gerandomiseerde fase II studie gepubliceerd in The New England Journal of Medicine, Deze deelstudie is onderdeel van de TOTARP-A studie die ook een deel B kent: http://www.icr.ac.uk/our-research/our-research-centres/clinical-trials-and-statistics-unit/clinical-trials/toparp
.gif)
Bedenk hierbij dat alle deelnemende patiënten (N=50) alle andere behandelingen voor prostaatkanker al hadden gehad, waaronder hormoonremmers, chemo met docetaxel en/of cabazitaxel, Abiraterone, enzalutamine enz. En dan alsnog een verdubbelde levensverlenging is echt opzienbarend.
Studie opzet en resultaten:
In deze studie werden 50 patiënten opgenomen en behandeld met olaparib (400 mg twee keer per dag) Alle patiënten waren eerder behandeld met docetaxel, 98% van de patiënten met abiraterone (Zytiga) of enzalutamide (Xtandi) en 58% met cabazitaxel.
Het primaire doel was de response, dus bij wie zou de olaparib nog iets doen, gedefinieerd als objectieve response, wanneer er een vermindering in de PSA - prostate-specific antigen level van gelijk of meer dan 50% zou worden gezien of een bevestigde vermindering van circulerende tumorcellen zoals geregistreerd in het bloed van ≥ 5 per 7.5 mL bloed tot <5/7.5 mL. Gerichte next-generation sequencing, exome en transcriptome analyses, en digitale polymerase chain reactie testen (= receptoren- en DNA onderzoek) werden uitgevoerd op bioptweefsel van alle patienten voordat de behandeling startte.
Response cijfers en overleving:
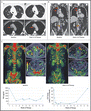
Overall response werd gezien bij 16 van de 49 evalueerbare patiënten (response rate = 33%, 95% confidence interval = 20%–48%). Next-generation sequencing identificeerde homozygous deletions, deleterious mutaties, of beide in de genen voor DNA-herstel, incl. BRCA1/2, ATM, Fanconi’s bloedarmoede genen, en CHEK2, kwamen voor bij 16 (335 afwijkingen) van de 49 patiënten. Van deze 16 hadden er 14 positieve biomarkers (88%, P < .001) versus patienten die een negatieve biomarkerscore hadden) en hadden een therapeutisch effect door de olaparib, inclusief alle 7 patiënten met een BRCA2 mutatie (vier met biallelic somatic verlies, drie met germline mutaties) en 4 van de 5 met ATM afwjikingen.
Mediane radiologische bevestigde progressievrije overleving was 9.8 maanden in patiënten met positieve biomarkers versus 2.7 maanden bij patienten (P < .001) met negatieve biomarkers. Mediane overall overleving was 13.8 maanden versus 7.5 maanden (P = .05) respectievelijk.
De meest voorkomende bijwerkingen van graad 3 en 4 waren bloedarmoede (20%) en vermoeidheid (12%).

Conclusie:
De onderzoekers concluderen: "Behandeling met de parpremmer olaparib bij patiënten welke hun prostaatkankertumoren niet langer meer reageren op standaardbehandelingen en die defecten lieten zien in genen voor DNA-herstel geeft hoge response cijfers".
Het volledige studierapport: DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer is gratis in te zien. Als u klikt op de video plaatje naast het abstract / studierapport kunt u in een korte video zien hoe olaparib werkt en hoe het wordt toegediend.
Hier het abstract van deze studie met referentielijst onderaan:
“Treatment with the PARP inhibitor olaparib in patients whose prostate cancers were no longer responding to standard treatments and who had defects in DNA repair genes led to a high response rate.”
Original Article
DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer
N Engl J Med 2015; 373:1697-1708 October 29, 2015 DOI: 10.1056/NEJMoa1506859
![]() Comments open through November 4, 2015
Comments open through November 4, 2015
Background
Prostate cancer is a heterogeneous disease, but current treatments are not based on molecular stratification. We hypothesized that metastatic, castration-resistant prostate cancers with DNA-repair defects would respond to poly(adenosine diphosphate –ribose) polymerase (PARP) inhibition with olaparib.
Methods
We conducted a phase 2 trial in which patients with metastatic, castration-resistant prostate cancer were treated with olaparib tablets at a dose of 400 mg twice a day. The primary end point was the response rate, defined either as an objective response according to Response Evaluation Criteria in Solid Tumors, version 1.1, or as a reduction of at least 50% in the prostate-specific antigen level or a confirmed reduction in the circulating tumor-cell count from 5 or more cells per 7.5 ml of blood to less than 5 cells per 7.5 ml. Targeted next-generation sequencing, exome and transcriptome analysis, and digital polymerase-chain-reaction testing were performed on samples from mandated tumor biopsies.
Results
Overall, 50 patients were enrolled; all had received prior treatment with docetaxel, 49 (98%) had received abiraterone or enzalutamide, and 29 (58%) had received cabazitaxel. Sixteen of 49 patients who could be evaluated had a response (33%; 95% confidence interval, 20 to 48), with 12 patients receiving the study treatment for more than 6 months. Next-generation sequencing identified homozygous deletions, deleterious mutations, or both in DNA-repair genes — including BRCA1/2, ATM, Fanconi’s anemia genes, and CHEK2 — in 16 of 49 patients who could be evaluated (33%). Of these 16 patients, 14 (88%) had a response to olaparib, including all 7 patients with BRCA2 loss (4 with biallelic somatic loss, and 3 with germline mutations) and 4 of 5 with ATM aberrations. The specificity of the biomarker suite was 94%. Anemia (in 10 of the 50 patients [20%]) and fatigue (in 6 [12%]) were the most common grade 3 or 4 adverse events, findings that are consistent with previous studies of olaparib.
Conclusions
Treatment with the PARP inhibitor olaparib in patients whose prostate cancers were no longer responding to standard treatments and who had defects in DNA-repair genes led to a high response rate. (Funded by Cancer Research UK and others; ClinicalTrials.gov number, NCT01682772; Cancer Research UK number, CRUK/11/029.)
-
References
-
1
Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin 2002;52:154-179
CrossRef | Web of Science | Medline
-
2
Baca SC, Prandi D, Lawrence MS, et al. Punctuated evolution of prostate cancer genomes. Cell 2013;153:666-677
CrossRef | Web of Science | Medline
-
3
Grasso CS, Wu Y-M, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012;487:239-243
CrossRef | Web of Science | Medline
-
4
de Bono JS, Ashworth A. Translating cancer research into targeted therapeutics. Nature 2010;467:543-549
CrossRef | Web of Science | Medline
-
5
Beltran H, Yelensky R, Frampton GM, et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol 2013;63:920-926
CrossRef | Web of Science | Medline
-
6
Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005;434:917-921
CrossRef | Web of Science | Medline
-
7
Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005;434:913-917
CrossRef | Web of Science | Medline
-
8
Fong PCP, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123-134
Free Full Text | Web of Science | Medline
-
9
Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-250
CrossRef | Web of Science | Medline
-
10
Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med 2012;366:1382-1392
Free Full Text | Web of Science | Medline
-
11
Ledermann J, Harter P, Gourley C, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014;15:852-861
CrossRef | Web of Science | Medline
-
12
Sandhu SK, Omlin A, Hylands L, et al. Poly (ADP-ribose) polymerase (PARP) inhibitors for the treatment of advanced germline BRCA2 mutant prostate cancer. Ann Oncol 2013;24:1416-1418
CrossRef | Web of Science | Medline
-
13
Sandhu SK, Schelman WR, Wilding G, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol 2013;14:882-892
CrossRef | Web of Science | Medline
-
14
Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol 2013;31:1748-1757
CrossRef | Web of Science | Medline
-
15
Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol 2008;26:1148-1159
CrossRef | Web of Science | Medline
-
16
Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-247
CrossRef | Web of Science | Medline
-
17
Robinson D, Van Allen EM, Wu Y-M, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215-1228
CrossRef | Web of Science | Medline
-
18
Ong M, Carreira S, Goodall J, et al. Validation and utilisation of high-coverage next-generation sequencing to deliver the pharmacological audit trail. Br J Cancer 2014;111:828-836
CrossRef | Web of Science | Medline
-
19
Carreira S, Romanel A, Goodall J, et al. Tumor clone dynamics in lethal prostate cancer. Sci Transl Med 2014;6:254ra125-254ra125
CrossRef | Web of Science
-
20
Scher HI, Heller G, Molina A, et al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J Clin Oncol 2015;33:1348-1355
CrossRef | Web of Science | Medline
-
21
McCabe N, Turner NC, Lord CJ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res 2006;66:8109-8115
CrossRef | Web of Science | Medline
-
22
Murai J, Huang SY, Das BB, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res 2012;72:5588-5599
CrossRef | Web of Science | Medline
-
23
Michels J, Vitale I, Saparbaev M, Castedo M, Kroemer G. Predictive biomarkers for cancer therapy with PARP inhibitors. Oncogene 2014;33:3894-3907
CrossRef | Web of Science | Medline
-
24
Ferraldeschi R, Nava Rodrigues D, Riisnaes R, et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur Urol 2015;67:795-802
CrossRef | Web of Science | Medline
-
25
Turenne GA, Paul P, Laflair L, Price BD. Activation of p53 transcriptional activity requires ATM’s kinase domain and multiple N-terminal serine residues of p53. Oncogene 2001;20:5100-5110
CrossRef | Web of Science | Medline
-
26
Beamish H, Kedar P, Kaneko H, et al. Functional link between BLM defective in Bloom’s syndrome and the ataxia-telangiectasia-mutated protein, ATM. J Biol Chem 2002;277:30515-30523
CrossRef | Web of Science | Medline
-
27
Canman CE, Lim DS, Cimprich KA, et al. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 1998;281:1677-1679
CrossRef | Web of Science | Medline
-
28
Miller KM, Tjeertes JV, Coates J, et al. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol 2010;17:1144-1151
CrossRef | Web of Science | Medline
-
29
Thurn KT, Thomas S, Raha P, Qureshi I, Munster PN. Histone deacetylase regulation of ATM-mediated DNA damage signaling. Mol Cancer Ther 2013;12:2078-2087
CrossRef | Web of Science | Medline
-
30
Adimoolam S, Sirisawad M, Chen J, Thiemann P, Ford JM, Buggy JJ. HDAC inhibitor PCI-24781 decreases RAD51 expression and inhibits homologous recombination. Proc Natl Acad Sci U S A 2007;104:19482-19487
CrossRef | Web of Science | Medline
-
31
Morris MJ, Molina A, Small EJ, et al. Radiographic progression-free survival as a response biomarker in metastatic castration-resistant prostate cancer: COU-AA-302 results. J Clin Oncol 2015;33:1356-1363
CrossRef | Web of Science | Medline
-
32
Kote-Jarai Z, Leongamornlert D, Saunders E, et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br J Cancer 2011;105:1230-1234
CrossRef | Web of Science | Medline
-
33
Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003;421:499-506
CrossRef | Web of Science | Medline
-
34
Chenevix-Trench G, Spurdle AB, Gatei M, et al. Dominant negative ATM mutations in breast cancer families. J Natl Cancer Inst 2002;94:205-215
CrossRef | Web of Science | Medline
-
35
Morgan SE, Lovly C, Pandita TK, Shiloh Y, Kastan MB. Fragments of ATM which have dominant-negative or complementing activity. Mol Cell Biol 1997;17:2020-2029
CrossRef | Web of Science | Medline
-
36
Barlow C, Eckhaus MA, Schäffer AA, Wynshaw-Boris A. Atm haploinsufficiency results in increased sensitivity to sublethal doses of ionizing radiation in mice. Nat Genet 1999;21:359-360
CrossRef | Web of Science | Medline
-
37
Buisson R, Dion-Côté A-M, Coulombe Y, et al. Cooperation of breast cancer proteins PALB2 and piccolo BRCA2 in stimulating homologous recombination. Nat Struct Mol Biol 2010;17:1247-1254
CrossRef | Web of Science | Medline
-
38
Chao OS, Goodman OB Jr. Synergistic loss of prostate cancer cell viability by coinhibition of HDAC and PARP. Mol Cancer Res 2014;12:1755-1766
CrossRef | Web of Science | Medline
-
39
Sternberg CN, Petrylak DP, Sartor O, et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol 2009;27:5431-5438
CrossRef | Web of Science | Medline
-
40
Fong PC, Yap TA, Boss DS, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol 2010;28:2512-2519
CrossRef | Web of Science | Medline
-
41
Ceccaldi R, O’Connor KW, Mouw KW, et al. A unique subset of epithelial ovarian cancers with platinum sensitivity and PARP inhibitor resistance. Cancer Res 2015;75:628-634
CrossRef | Web of Science | Medline
-
Gerelateerde artikelen
- Prostaatkankerpatienten waarbij DNA is getest via in bloed circulerend tumorweefsel (ctDNA) hebben bij positieve BRCA-1 en 2 en ATM mutaties meer baat bij olaparib in vergelijking met enzalutamide en abiraterone
- Olaparib geeft hoge respons - 33 procent en verdubbelt mediane overleving van 7,5 naar 13,8 maanden bij mannen met zwaar voorbehandelde hormoonresistente uitgezaaide prostaatkanker. copy 1
- Olaparib heeft alleen goed effect bij mannen met uitgezaaide prostaatkanker met het BRCA 1 of 2 mutatie. Mannen met ATM mutaties reageerden nauwelijks op olaparib.
- Olaparib geeft betere resultaten op ziekteprogressievrije tijd en overall overleving dan enzalutamide en abiraterone bij patienten met uitgezaaide hormoonresistente prostaatkanker met de juiste DNA mutaties.



Plaats een reactie ...
Reageer op "Olaparib geeft hoge respons - 33 procent en verdubbelt mediane overleving van 7,5 naar 13,8 maanden bij mannen met zwaar voorbehandelde hormoonresistente uitgezaaide prostaatkanker. copy 1"