Update artikel 17 oktober 2016:
Lees ook:
https://kanker-actueel.nl/NL/ldn-low-dose-naltrexone.html
Hier links naar enkele studies met LDN - Low Dose Naltrexon. Klik op de links en u komt bij de studierapporten en zie ook gerelateerde artikelen:
Opioid growth factor improves clinical benefit and survival in patients with advanced pancreatic cancer
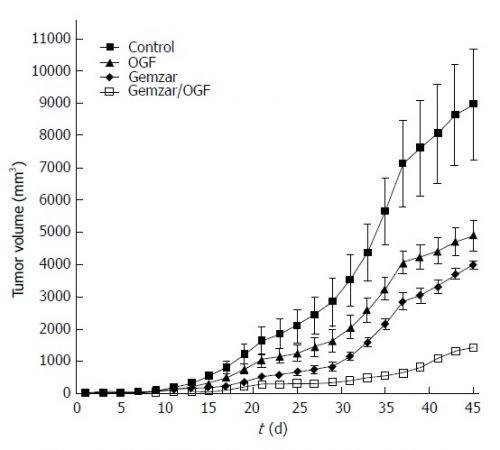
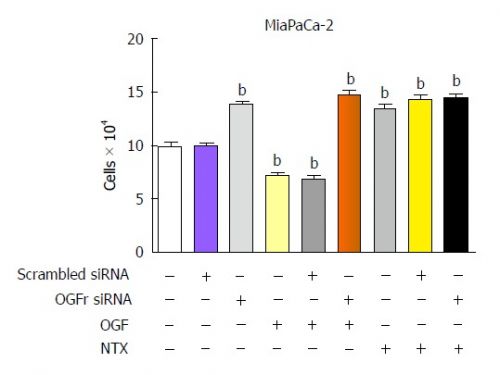
Grafiek van effecten van LDN bij gemzar bij alvleesklierkanker
Conclusion
OGF biotherapy improves the clinical benefit and prolongs survival in patients with pancreatic cancer by stabilizing disease or slowing progression. The effects of OGF did not adversely alter patient quality of life. The use of OGF biotherapy at earlier stages of disease or in combination with other chemotherapeutic agents may further improve the outcome of this malignancy.
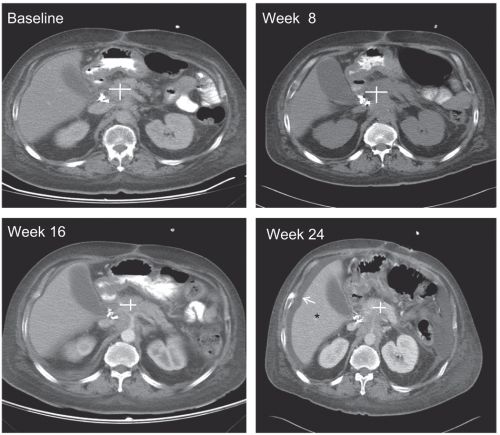
Scanbeelden tijdens behandeling van LDN bij alvleesklierkankerpatienten
Opioid growth factor and the treatment of human pancreatic cancer: a review.
Conclusion:
Clinically, OGF is a safe, non-toxic biotherapy that extends survival and reduces tumor burden in patients with unresectable pancreatic cancer. In summary, the OGF-OGFr axis should be explored both as a primary therapy for pancreatic cancer, and as an adjuvant pathway with other chemotherapies.
Grafiek boven: LDN bij alvleesklierkanker
Long-term remission of adenoid cystic tongue carcinoma with low dose naltrexone and vitamin D3--a case report.
Low-dose naltrexone therapy improves active Crohn's disease.
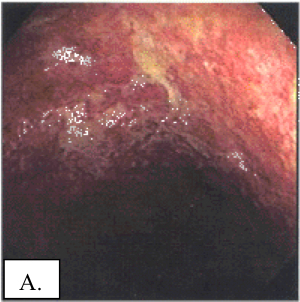
A: Voor de behandeling met LDN
B: na de behandeling met LDN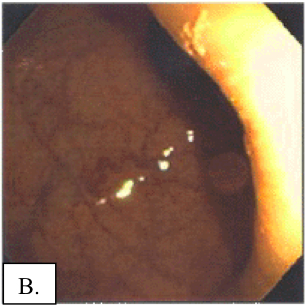
Low-dose naltrexone suppresses ovarian cancer and exhibits enhanced inhibition in combination with cisplatin.
The biology of the opioid growth factor receptor (OGFr).
Diabetic keratopathy and treatment by modulation of the opioid growth factor (OGF)-OGF receptor (OGFr) axis with naltrexone: a review.
Selective blockade of the OGF-OGFr pathway by naltrexone accelerates fibroblast proliferation and wound healing.
Mei 2002:
Naar naltrexone is wel onderzoek gedaan bij muizen ingespoten met menselijke kankercellen. Hier wordt een overzicht gegeven van wat er tot nu toe aan onderzoeksgegevens bekend zijn. Op de site kun je ook de abstract allemaal aanklikken en lezen. De nummers verwijzen naar de studies/referenties zoals die achter deze beschrijving zijn gegeven
- Research History
Ian Zagon, Ph.D., whose research group has done much of the basic animal work in the area of cancer treatment and endorphins, showed in 1981 in a mouse neuroblastoma model that very small doses (0.1 mg./kg) of naltrexone, given once a day, inhibit tumor growth, prolong survival in those mice that develop tumors and protect some mice from developing tumors altogether.2, 3 (abstract)
Zagon had hypothesized that the small daily doses of naltrexone work to enhance the endorphin-related protective effect against cancer in mice by increasing the number and density of opiate receptors on tumor cells. He hypothesized as well that the increase in endorphins known to be induced by naltrexone might play a part in the protective effect of the small daily dose by working directly on the tumors' opiate receptors.4 (abstract)
In 1996 and 1997, Zagon and his co-workers, reported on laboratory research using specially-bred mice that had no immune system (so-called "nude mice"). They transplanted, in separate experiments, human colon cancer and human pancreatic cancer into the animals and compared the growth of the cancer between those mice that received daily injections of metenkephalin and a control group that received placebo. In each experiment, metenkephalin acted as a negative regulator of tumorigenesis and was significantly able to suppress tumor appearance and growth in the treated group.5 (abstract)
Of especial importance, in 1996 the same group of researchers demonstrated that by utilizing LDN to induce an intermittent blockade of opioid receptors in similar laboratory animals (nude mice), the growth of inoculated human colon cancer was markedly retarded. "At the time (10 days) when all control mice had tumors, 80% of the mice in the 0.1 mg/kg NTX group had no signs of neoplasia." When measurements of metenkephalin plasma levels were made, the group that received LDN had metenkephalin levels that were elevated 2.5-fold compared with the control group. The researchers concluded that the results suggested "that daily intermittent opioid receptor blockade with NTX [low dose naltrexone] provokes the interaction of opioids and receptors in the interval following drug availability, with opioids serving to inhibit tumorigenicity of human colon cancer".6 (abstract)
New findings by Zagon and colleagues at The Pennsylvania State University in Hershey were published in the December 1999 issue of the journal Brain Research. They had identified the specific cell receptor for one of the endorphins, metenkephalin (the levels of which are increased by LDN). Zagon stated that the opioids act as growth inhibitors, as well as neurotransmitters, and that this feature has implications for cancer treatment. Metenkephalin is found in all tissues, and appears to be associated with cells undergoing renewal or proliferation. Zagon's group was described as having mounted Phase I trials using metenkephalin in an attempt to control the growth of pancreatic cancer in humans. Pancreatic tumors appear to have low levels of the metenkephalin receptor. Low peptide or receptor levels may exist in cancer cells in general since they want to stimulate their own growth, Zagon said.7 (abstract) - De referenties zoals hier genoemd, en zoals gezegd op de site kunnen de abstracts direct worden aangeklikt.:
- Referenties:
Matthew, PM, Froelich CJ, Sibbitt WL, Jr., Bankhurst AD, Enhancement of natural cytotoxicity by beta-endorphin, J Immunol 130, pp.1658-1662, Apr 1983. Read the abstract. - Zagon IS, McLaughlin PJ, Naltrexone prolongs the survival time of mice treated with neuroblastoma, Life Sci 28, pp. 1095-1102, 1981. (Abstract unavailable.)
- Zagon IS, McLaughlin PJ, Naltrexone modulates tumor response in mice with neuroblastoma, Science 221, pp.671-3, Aug 12, 1983. Read the abstract.
- Hytrek SD, McLaughlin PJ, Lang CM, Zagon IS, Inhibition of human colon cancer by intermittent opioid receptor blockade with naltrexone, Cancer Lett 101(2), pp. 159-64, Mar 29, 1996. Read the abstract.
- Zagon IS, Hytrek SD, Lang CM, Smith JP, McGarrity TJ, Wu Y, McLaughlin PJ, Opioid growth factor (enkephalin) prevents the incidence and retards the growth of human colon cancer, Am J Physiol 271(3 Pt 2), pp.R780-R786, Sep 1996. Read the abstract.
- Hytrek SD, et al. 1996. Zagon IS, Verderame MF, Allen SS, McLaughlin PJ, Cloning, sequencing, expression and function of a cDNA encoding a receptor for the opioid growth factor, [Met(5)]enkephalin, Brain Res 849(1-2), pp. 147-54, Dec 4, 1999. Read the abstract.
- Other References
Recant L, Voyles NR, Luciano M, Pert CB, Naltrexone reduces weight gain, alters "beta-endorphin", and reduces insulin output from pancreatic islets of the genetically obese mice, Peptides1(4), pp. 309-313, Winter 1980. Read the abstract.
Zagon IS, McLaughlin PJ., Opioid antagonists inhibit the growth of metastatic murine neuroblastoma, Cancer Letters 21, pp. 89-94, 1983. Read the abstract.
Zagon IS, McLaughlin PJ, Duration of opiate receptor blockade determines tumorigenic response in mice with neuroblastoma: a role for endogenous opioid systems in cancer, Life Sci 35, pp. 409-416, 1984. Read the abstract.
Zagon IS, McLaughlin PJ, Opioid antagonist modulation of murine neuroblastoma: A profile of cell proliferation and opioid peptides and receptors, Brain Res 480, pp. 16-28, 1989. Read the abstract.
Zagon IS, Hytrek SD, Smith JP, McLaughlin PJ, Opioid growth factor (OGF) inhibits human pancreatic cancer transplanted into nude mice, Cancer Lett 112(2), pp.167-175, Jan 30, 1997. Read the abstract.
Referentielijst van studies met LDN - Low Dose Naltrexone bij alvleesklierkanker
References
Gerelateerde artikelen
- Naltrexone blijkt een prima middel bij auto-immuunziektes. Zie hier laatste studieresultaten bij o.a. ziekte van Crohn, MS - Multiple Sclerose en uitleg wat LDN - Low Dose Naltrexone precies is.
- Het werkingsmechanisme van Naltrexone
- Onderzoeksgeschiedenis van Naltrexone
- Beschrijving van 242 patienten die naltrexone kregen als aanvullende behandeling.
- Voorbeelden van patiënten die Naltrexone gebruiken.
- LDN - Low Dose Naltrexone is een niet toxisch middel dat voor enkele toepassingen goedgekeurd is door de FDA



Plaats een reactie ...
Reageer op "Onderzoeksgeschiedenis van Naltrexone"