Aan dit artikel is enkele uren gewerkt. Mocht u ons willen helpen kanker-actueel online te houden dan kan dat met een machtiging of directe donatie via: http://kanker-actueel.nl/NL/donaties.html of al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Terneuzen.
9 oktober 2017: Bron: BMJ 2017;359:j4530
Slechts de helft van de door de European Medicines Agency (EMA) officieel goedgekeurde nieuwe kankermedicijnen geven een mediane verbetering van de overall overleving. Maar die verbetering, als die er al is, blijkt marginaal met een mediane verbetering van overall overleving van 2,7 maanden. (variërend van 1 tot 5,8 maanden). Verbetering van kwaliteit van leven werd in de 23 studies waar dit ook als doel stond bereikt bij 48% (11 uit 23).
De Engelse onderzoekers, Courtney Davis en collega's zijn hard in hun conclusie: veel medicijnen worden beoordeeld en goedgekeurd op basis van ondeugdelijke resultaten of studiedoelen die geen goede voorspellers blijken voor de mate van overleving en/of kwaliteit van leven.
Conclusions: This systematic evaluation of oncology approvals by the EMA in 2009-13 shows that most drugs entered the market without evidence of benefit on survival or quality of life. At a minimum of 3.3 years after market entry, there was still no conclusive evidence that these drugs either extended or improved life for most cancer indications. When there were survival gains over existing treatment options or placebo, they were often marginal.
De studieresultaten zijn afgelopen week gepubliceerd in BMJ.
In de periode 2009 tot en met 2013 gaf de EMA goedkeuring aan 48 kankermedicijnen voor 68 indicaties. Bv. olaparib werd goegekeurd voor zowel borstkankerpatiënten als eierstokkankerpatiënten met een BRCA 1 / 2 mutatie. Bij 35 van de 68 indicaties bij een mediane follow-up van 5,4 jaar vonden de onderzoekers van deze nieuwe studie statistisch significante verbetering van de overleving of verbetering van de kwaliteit van leven. De medicatie voor de andere 33 indicaties liet geen verbetrring van de overall overleving zien en ook geen verbeterde kwaliteit van leven.
Hier een grafiek van de onderzochte medicijnen voor de 68 indicaties. Teskt gaat onder grafiek verder.
Characteristics of approvals of cancer drug, 2009-13. Figures are numbers (percentage) of indications
| Characteristics | Solid tumours (n=51) | Haematological tumours (n=17) |
|---|---|---|
| Type of marketing authorisation: | ||
| First marketing authorisation | 24 (47) | 9 (53) |
| Extension | 27 (53) | 8 (47) |
| Pathway of first marketing authorisation: | ||
| Regular approval | 19 (79) | 4 (44) |
| Conditional approval | 5 (21) | 5 (56) |
| Orphan designation | 3 (6) | 8 (47) |
| Intent of treatment: | ||
| Curative | 6 (12) | 1 (6) |
| Non-curative | 45 (88) | 16 (94) |
Wat de onderzoekers vooral benadrukken in hun studie is dat geen enkele studie die beslissend was voor de offciële goedkeuring en dus toelating tot de klinische praktijk als doel had ‘kwaliteit van leven’, zelfs niet als het onderzochte middel vooral palliatief werd ingezet. In iets meer dan de helft van de indicaties was kwaliteit van leven een secundair doel van het onderzoek, maar de resultaten daarvan waren echt heel slecht: slechts in zeven studies was er sprake van een statistisch significante verbetering van de kwaliteit van leven.
Deze medicijnen gaven uiteindelijk een verbetering van kwaliteit van leven. Ik heb dit niet vertaald. Anders gebruik google translationknop rechtsboven dit artikel.
Box 2: Cancer drugs associated with benefit on health related quality of life (HRQoL)
Reported at time of market approval
-
Afatinib v pemetrexed/cisplatin (for the treatment of TKI-naive, EGFR mutation +ve, non-small cell lung cancer) significantly delayed the time to deterioration for cough and dyspnoea but not time to deterioration of pain. HRQoL was evaluated against prespecified components of the European Organisation for Research and Treatment of Cancer quality of life questionnaire C30 (EORTC QLQ-C30) questionnaire and lung cancer module QLQ-LC13. The study was open label43
-
Gefitnib (for the treatment of metastatic non-small cell lung cancer) showed significant benefits on quality of life compared with carboplatin plus paclitaxel, and with docetaxel on some measures, but not compared with placebo. HRQoL was assessed with the functional assessment of cancer therapy-lung (FACT-L), and trial outcome index (TOI), and symptom improvement by the lung cancer subscale (LCS). The active comparator trials were open label. The trial v placebo was double blind44
-
Tegafur, gimeracil, oteracil (S-1) (in combination with cisplatin for the treatment of advanced gastric cancer) showed a significant improvement over cisplatin/5-fluorouracil for only one of the subscales (physical wellbeing) of the functional assessment of cancer therapy-gastric (FACT-Ga) HRQOL instrument. The study was open label45
-
Vandetanib v placebo (for the treatment of metastatic medullary thyroid cancer) significantly improved “time to worsening of pain” (a composite endpoint based on patient reported analgesic use and responses to the brief pain inventory) but did not show a significant improvement for the functional assessment of cancer therapy-general (FACT-G) score. The study was double blind46
Reported in the postmarketing period
-
Crizotinib (for second line treatment of ALK mutation +ve advanced non-small cell lung cancer) showed significant improvements compared with pemetrexed across a range of HRQoL measures, including a significantly greater delay in time to worsening of symptoms. HRQoL was assessed with the European Organisation for Research and Treatment of Cancer quality of life questionnaire C30 (EORTC QLQ-C30) and the lung cancer module QLQ-LC13. The study was open label47
-
Erlotinib (for first line treatment of EGFR mutation +ve metastatic non-small cell lung cancer) showed clinically relevant and significant improvements compared with gemcitabine/carboplatin in total functional assessment of cancer therapy-lung (FACT-L), trial outcome index (TOI), and lung cancer subscale (LCS) scores. The study was open label48
-
Nilotinib v imatinib (for patients with newly diagnosed Ph+ chronic myeloid leukaemia) reported a significant quality of life improvement with respect to some categories of low grade adverse events. HRQoL was assessed with generic SF-36 and leukaemia specific functional assessment of cancer therapy-leukaemia (FACT-Leu) surveys. The study was open label42
-
Ofatumumab (in patients with chronic lymphocytic leukaemia refractory to fludarabine and alemtuzumab) showed significant improvements v “physician’s choice” with respect to fatigue but not side effects of treatment or effects of disease. HRQoL was evaluated according to three prespecified domains of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC-C30) and the chronic lymphocytic leukaemia module QLQ-CLL16 (fatigue, side effects of treatment, and effects of disease). The study was open label49
-
Pazopanib (for first line treatment of advanced renal cell carcinoma) significantly improved HRQoL compared with sunitinib with respect to 11 of 14 HRQoL domains. HRQoL was assessed with the functional assessment of chronic illness therapy-fatigue (FACIT-F) questionnaire, the National Comprehensive Cancer Network/functional assessment of cancer therapy-kidney symptom index 19 (NCCN-FACT FKSI-19), and the cancer therapy satisfaction questionnaire. The study was open label50
Het overzicht van de belangrijkste onderzochte medicijnen, is wel klein maar hopelijk nog wel te lezen:
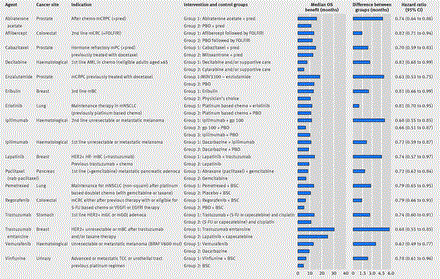
Fig 1 Magnitude of overall survival benefit at the time of EMA approval. Figure excludes seven indications for which median overall survival could not be estimated at time of marketing authorisation: mifamurtide for resectable non-metastatic osteosarcoma after complete resection (+chemo); pertuzumab for 1st line HER2+mBC; pomalidomide for 3rd line (+dexamethasone) relapsed and refractory multiple myeloma; rituximab for 1st line CLL (+chemo); trastuzumab for HER2+ BC (+taxane) after adjuvant chemo; trastuzumab for HER2+BC (+adjuvant chemo); and trastuzumab for HER2+locally advanced BC (+neoadjuvant chemo and as monotherapy adjuvantly). Box 1 shows abbreviations used
Hier een grafiek van de efectiviteit van de goedgekeurde medicijnen nadat zij op de markt kwamen:
Fig 2 Availability of benefits on overall survival and quality of life of cancer drugs approved 2009-13. Box 1 shows abbreviations used

Deborah Cohen, verbonden aan BMJ, heeft in de studies die zij beoordeelden een heleboel fouten geconstateerd, zoals methodologische fouten in het ontwerp, de uitvoering, de analyse en de verslaglegging. Die zijn volgens haar allemaal door de EMA over het hoofd gezien.
Dat medicijnen op ondeugdelijke gronden worden toegelaten, heeft volgens Deborah Cohen ernstige gevolgen. Zij geeft twee voorbeelden. De eerste: een publicatie met statistisch hoopgevende resultaten leidt tot onrealistische verwachtingen bij kankerpatiënten en ook bij oncologen. En het tweede uunt dat Deborah Cohen maakt: het ontbreken van een goed onderbouwd bewijs van de effectiviteit brengt de betalende instanties en regeringen in een moeilijk parket in hun onderhandelingen met de producenten van de medciijnen. Wat te doen met de financiering van deze dure geneesmiddelen?
Al met al dus een ontluisterend beeld. Dat alleen maar bevestigd dat de kankerpatient zelf totaal onbelangrijk is in het wetenschappelijke onderzoek
Het volledige studierapport: Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13 is gratis in te zien.
Hier het abstract met referentielijst:
This systematic evaluation of oncology approvals by the EMA in 2009-13 shows that most drugs entered the market without evidence of benefit on survival or quality of life. At a minimum of 3.3 years after market entry, there was still no conclusive evidence that these drugs either extended or improved life for most cancer indications. When there were survival gains over existing treatment options or placebo, they were often marginal.
Availability of evidence of benefits on overall survival and quality of life of cancer drugs approved by European Medicines Agency: retrospective cohort study of drug approvals 2009-13
BMJ 2017; 359 doi: https://doi.org/10.1136/bmj.j4530 (Published 04 October 2017) Cite this as: BMJ 2017;359:j4530
- Courtney Davis, senior lecturer1,
- Huseyin Naci, assistant professor of health policy2,
- Evrim Gurpinar, MSc candidate in international health policy 2,
- Elita Poplavska, assistant professor3,
- Ashlyn Pinto, MSc candidate in global health2,
- Ajay Aggarwal, academic clinical oncologist4 5
- 1Department of Global Health and Social Medicine, King’s College London, London WC2R 2LS, UK
- 2LSE Health, Department of Health Policy, London School of Economics and Political Science, London, UK
- 3Faculty of Pharmacy, Riga Stradins University, Riga, Latvia
- 4Department of Health Services Research and Policy, London School of Hygiene and Tropical Medicine, London, UK
- 5Institute of Cancer Policy, King’s College London, London, UK
- Correspondence: C Davis courtney.davis@kcl.ac.uk
- Accepted 28 September 2017
Abstract
Objective To determine the availability of data on overall survival and quality of life benefits of cancer drugs approved in Europe.
Design Retrospective cohort study.
Setting Publicly accessible regulatory and scientific reports on cancer approvals by the European Medicines Agency (EMA) from 2009 to 2013.
Main outcome measures Pivotal and postmarketing trials of cancer drugs according to their design features (randomisation, crossover, blinding), comparators, and endpoints. Availability and magnitude of benefit on overall survival or quality of life determined at time of approval and after market entry. Validated European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) used to assess the clinical value of the reported gains in published studies of cancer drugs.
Results From 2009 to 2013, the EMA approved the use of 48 cancer drugs for 68 indications. Of these, eight indications (12%) were approved on the basis of a single arm study. At the time of market approval, there was significant prolongation of survival in 24 of the 68 (35%). The magnitude of the benefit on overall survival ranged from 1.0 to 5.8 months (median 2.7 months). At the time of market approval, there was an improvement in quality of life in seven of 68 indications (10%). Out of 44 indications for which there was no evidence of a survival gain at the time of market authorisation, in the subsequent postmarketing period there was evidence for extension of life in three (7%) and reported benefit on quality of life in five (11%). Of the 68 cancer indications with EMA approval, and with a median of 5.4 years’ follow-up (minimum 3.3 years, maximum 8.1 years), only 35 (51%) had shown a significant improvement in survival or quality of life, while 33 (49%) remained uncertain. Of 23 indications associated with a survival benefit that could be scored with the ESMO-MCBS tool, the benefit was judged to be clinically meaningful in less than half (11/23, 48%).
Conclusions This systematic evaluation of oncology approvals by the EMA in 2009-13 shows that most drugs entered the market without evidence of benefit on survival or quality of life. At a minimum of 3.3 years after market entry, there was still no conclusive evidence that these drugs either extended or improved life for most cancer indications. When there were survival gains over existing treatment options or placebo, they were often marginal.
-
Transparency: The lead author affirms that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
References
Gerelateerde artikelen
- Lees hier hoe de Vereniging tegen Kwakzalverij, VtdK, de weg helemaal is kwijtgeraakt afgelopen jaren. Een overzicht van artikelen en publicaties
- Academische uitgevers aangeklaagd wegens uitbuiting van wetenschappers. Uitgevers verdienden in 2023 miljarden aan publicaties die uitgevoerd zijn met belastinggeld
- Yvan Wolffers overlijdt aan de gevolgen van prostaatkanker op 7 oktober 2022
- VITALITY OF LIFE BY PERSONALIZED MEDICINE Bruggen bouwen in het complementaire veld. 2 daags jubileumcongres om het 40-jarig bestaan van de NatuurApotheek en het 20+2 jarig bestaan van de Hahnemann Apotheek op 2 en 3 september 2022
- Ministerie van Economische Zaken en Klimaat (EZK) liet advies minder vlees te eten bewust weg uit klimaatcampagne, zo ontdekte Stichting Wakker Dier
- Nieuwe kankermedicijnen worden in Europa (EMA) veel later goedgekeurd dan in Amerika (FDA) en kost tienduizenden levensjaren.
- Wetenschappers van het Anthonie van Leeuwenhoek ziekenhuis zeggen met combinatiebehandeling (one-two-punch ) van reeds bestaande medicijnen de sleutel tot genezende behandeling van kanker te hebben gevonden
- Nieuw NANO-Vaccin, 2 natuurlijke peptiden , aminozuren verpakt in nanodeeltjes voorkomt melanomen en uitzaaiingen bij melanomen en blijkt als immuuntherapie uitstekend te werken
- Whole genome sequencing: toekomst van de kankerdiagnostiek? AvL start studie samen met UMC Utrecht
- Surviving terminal cancer: kunnen overlevenden van een hersentumor helpen kanker te overwinnen? Drie mannen overwonnen hun hersentumor - GBM - en 2 ervan zijn al 15 en 20 jaar vrij van kanker met eigen cocktail van bewezen niet-toxische middelen.
- AMC start studieproject EIGEN ONDERZOEK waarbij 500 patienten met darmklachten en chronische vermoeidheid zelf hun ervaringen met probiotica en beweging enz. bijhouden via een app.
- Chemo stimuleert juist kankergroei en uitzaaiïngen door verhoogde productie van het eiwit WNT16B dat ook grote rol speelt bij wondheeling
- Kanker opsporen binnen 20 minuten en een kant en klaar behandelingsvoorstel wordt binnen drie jaar mogelijk met nieuw apparaat gebaseerd op nanotechnologie.
- Effecten van medicijnen en behandelingen bij 52 aandoeningen waaronder kanker moeten volgens het Zorginstituut vermeld worden in persoonlijke medische dossiers
- vorinostat heft resistentie van kankercellen op bij melanomen en lijkt oplossing voor dure medicijnen aldus Rene Bernards van het NKI. Vorinostat blijkt ook bij recidief van zwaar voorbehandelde multiple myeloma met 94 procent bijzonder effectief
- Keer diabetes om wint zinnige zorg award voor voedingsprogramma voor diabetici
- E-sigaret blijkt 95 procent minder schadelijk dan tabak roken en stimuleert rokers te stoppen. Echter tieners lijken er juist door gestimuleerd te worden te beginnen met roken
- Zorginstituut ziet verdere verbetering van behandelingen van niercelkanker met nieuwe medicijnen en doet aanbevelingen in een rapport aan Minister Bruins.
- Bepaalde darmbacteriën kunnen de effectiviteit van immunotherapie met anti-PD medicijnen verhogen bij de behandeling van melanomen
- Mammografie zorgt nauwelijks voor vermindering van risico op overlijden aan gevorderde borstkanker maar wel voor veel meer onnodige behandelingen door overdiagnose
- Vitamine C in combinatie met Doxycycline een antibiotica doden samen kankerstamcellen en lijkt nieuwe behandelingsoptie nagenoeg zonder bijwerkingen
- Antibiotica verminderen effectiviteit van immuuntherapie terwijl aanvullende microbiota de resultaten sterk verbeteren van immuuntherapie met anti-PD medicijnen
- Goedkeuring van nieuwe kankermedicijnen meestal gebaseerd op te weinig bewijs en geven in de praktijk slechts bij de helft kleine verbeteringen in mediane overleving.
- Biliscreen, een speciale app kan via een selfie van oog vroegtijdig alvleesklierkanker en aan geelzucht gerelateerde leveraandoeningen ontdekken.
- Nederlandse DRUP studie, waarbij behandelingen worden gegeven gebaseerd op DNA mutaties en receptorenexpressie geeft hoopvolle resultaten voor uitbehandelde kankerpatienten
- Immuuntherapeutische studies zoals gepresenteerd Op ASCO 2017 in Chicago 2 t/m/ 5 juni 2017
- Propranolol, een bloeddrukverlager, blijkt effectief geneesmiddel tegen angiosarcomen en is goedgekeurd als weesgeneesmedicijn voor onderzoek door Europese Commissie.
- Darmflora met gevarieerde bacterien geven betere resultaten voor immuuntherapie bij melanomen dan een minder gevarieerde darmflora.
- Zuurstof tekort bevordert groei kankercellen. Toevoeging van zuurstof - ozontherapie - kan groei van tumorcellen remmen of zelfs tegengaan
- Melkzuurbacterien - probiotica aanwezig in gezond borstweefsel beschermt vrouwen tegen borstkanker blijkt uit kleinschalige studie
- The quest for the cures of cancer, bekijk documentaire van oncologen, wetenschappers en patienten over natuurlijke geneeswijzen bij kanker
- Vader Pieter en zoon Bernard van Vollenhoven genezen van uitgezaaide melanoom en lymfklierkanker. Ook Jimmy Carter blijkt kankervrij. Bepalen geld en macht de kansen op genezen van kanker?
- Gepersonaliseerd vaccin voor immuuntherapie is de sleutel tot voorkomen en genezen van kanker ontdekt een team van wetenschappers.
- LUMC opent Nederlandse Donor Feces Bank (NDFB) - poepbank waar iedereen die gezond is zijn ontlasting kan doneren, welke gebruikt wordt voor herstel van darmflora bij zieke patienten.via neussonde
- In Nederland overlijden elk jaar de meeste mensen onder de 65 jaar aan kanker en Nederland staat tweede voor mensen ouder dan 65 jaar van heel Europa
- Dr. Nicolas Gonzalez sterft onder verdachte omstandigheden. Dr. Gonzalez is in 1 jaar de tijd de 10e complementair werkende arts die overlijdt door moord of onder verdachte omstandigheden
- Nieuwe aanpak van kanker gevonden door Amerikaanse tiener?
- PLEKHA7 een eiwit speelt cruciale rol in apoptose - zefldoding van kankercellen. Onderzoekers aan de Mayo Clinic ontdekken dat reparatie van PLEKHA7 vorming van tumoren kan voorkomen.
- Doorbraak in bestrijden van chronische pijn met de lichaamseigene ontstekingsremmende stof palmitoylethanolamide welke geen bijwerkiingen geeft
- Darmkankeronderzoek: de eerste resultaten gepresenteerd. Bij 1 op de 28 werd iets afwijkends gevonden
- ZONmw pleit in rapport voor meer integratie van alternatieve, complementaire, aanvullende middelen en behandelingen in de reguliere gezondheidszorg
- Nano-MRI met nanovloeistof ontdekt sneller uitzaaiingen in lymfklieren en kan behandeling van kanker sneller doen starten of juist aantonen dat behandelingen niet nodig zijn.
- Welke behandeling krijgt de patiente met dikke darmkanker uit de Wereld Draait Door van vanavond 8 oktober 2013?
- Nieuwe Tieten van Sacha Polak. is een persoonlijke en openhartige documentaire over wel of niet preventief haar borsten te laten verwijderen omdat.ze draagster is van het BRCA-1 gen
- Mamma waarom moet je naar het ziekenhuis? Nieuwe animaties en tekeningen voor peuters en kinderen die een ouder met kanker hebben op website van stichting Kankerspoken.
- Erasmus Medisch Centrum gaat onderzoek doen naar effecten van hyperthermie bij kanker als aanvulling op chemo of bestraling
- Injecties met 3-bromopyruvate (3-BrPA) in tumorweefsel zou effectiever werken dan operatie en chemo bij borstkanker..Deze antiglycolitic therapie beinvloed metabolisch proces in kankercel en leidt tot natuurlijke celdood
- Time released chemo toedienen via nanodeeltjes bij hersentumoren - glioma blastoma multiforme, verdubbelt overlevingstijd in dierstudies
- Op menselijke genen kan geen patent worden verkregen zo luidt het oordeel van het Hooggerechtshof in de Verenigde Staten. Op synthetische DNA-sequenties mag dit echter wel.
- In Nederland bestaat er grote ongelijkheid in gebruik van dure diagnostiek en behandelingen voor kankerpatienten, aldus prof. dr. Carin Uyl-de Groot.
- Botuitzaaiingen worden vaak veroorzaakt door stamcellen in het beenmerg, ontdekken wetenschappers.
- Nieuwe vorm van uitstrijkje zou vroegtijdig eierstokkanker, baarmoederkanker en baarmoederhalskanker ontdekken, beweren Amerikaanse onderzoekers
- BG-12 - dimethyl fumaraat - verbetert significant kwaliteit van leven en vermindert klachten van bepaalde vorm van MS - multiple sclerose (RR MS = de schubvorm van MS), aldus meta analyse van fase III studies.
- Darmkanker stijgt met 42 procent de laatste tien jaar. Wereld Kanker Onderzoek Fonds start groot internationaal onderzoek naar effecten van leefstijl en voeding op risico van darmkanker
- Voedingstoffen als medicijn: biochemische kaart van menselijk lichaam voltooid. Ziektes kunnen voorkomen worden door individueel voedingspatroon.
- Bloedtest kan bepalen of er chemo nodig is bij uitgezaaide borstkanker en darmkanker, aldus promotie onderzoek van Bianca Mostert



Plaats een reactie ...
Reageer op "Goedkeuring van nieuwe kankermedicijnen meestal gebaseerd op te weinig bewijs en geven in de praktijk slechts bij de helft kleine verbeteringen in mediane overleving."