12 februari 2023: zo nu en dan krijg ik een vraag over het CIMAvax vaccin voor longkanker uit Cuba. Afgelopen week kreeg ik weer een vraag daarover en ben eens gaan zoeken op internet hoe het er nu voorstaat.
Dit artikel geeft weer hoe longkankerpatiënten in de klinische praktijk reageren op het CIMEvax vaccin, waarbij aangetekend dat er geen EGFR-mutatie wordt gemeten vooraf. Dus deze resultaten gaan over alle longkankerpatiënten waarvan bijna iedereen in stadium IIIB of stadium IV zat voordat begonnen werd met deze vorm van immuuntherapie.
Klik op de titel van het abstract dat onderaan artikel volledig staat, maar lees ook verder in dit artikel voor meer informatie.
Safety and efficacy of CIMAvax-EGF vaccine for the treatment of real-world non-small cell lung cancer patients
22 april 2022: Zie ook dit artikel: https://kanker-actueel.nl/specifieke-bloedwaarden-en-biomarkers-die-wijzen-op-veroudering-van-immuunsysteem-immunosenescentie-biomarkers-plus-basale-egf-concentratie-in-bloed-resulteerden-in-goede-voorspellers-van-het-succes-van-het-cimavax-egf-vaccin-bij-gevorderde-longkanke.html
17 maart 2021: Aanvullend op onderstaande informatie is deze studie naar de werking van het CIMAvax vaccin bij longkankerpatiënten interessant. Het gaat om deze studie: (clinical trial registration number: RPCEC00000161, http://registroclinico.sld.cu/).
Het Cimavax-EGF vaccin blijkt bij de longkankerpatiënten uit een fase III studie veel antistofffen te genereren tegen de EGFR mutatie, vooral bij degenen waar het vaccin goed aansloeg waren er veel antistoffen en van goede kwaliteit gemeten. Kanker met een EGFR mutatie is in het algemeen moeilijker te behandelen dan vormen van kanker zonder EGFR mutatie of met andere mutaties.
Officiele conclusie uit de studie: het CIMAvax-EGF-vaccin induceert een EGF-specifieke beschermende humorale respons bij een hoog percentage van de gevaccineerde patiënten met niet-kleincellige longkanker, waarvan de hoeveelheid en kwaliteit geassocieerd waren met klinisch voordeel.
Zie het hele studierapport via onderstaande link. Het abstract staat onderaan dit artikel
Anti-EGF antibodies as surrogate biomarkers of clinical efficacy in stage IIIB/IV non-small-cell lung cancer patients treated with an optimized CIMAvax-EGF vaccination schedule
14 augustus 2017: Bron: Frontiers in immunology (en met dank aan Marina)
Meer dan 4000 mensen met kanker zouden zijn geholpen door het Cubaanse virus CIMAvax. Dat inmiddels goedgekeurd is om te gebruiken bij longkanker en daarmee ook uitstekende resultaten heeft laten zien. Nu blijkt uit een aanvullende fase IV studie na een fase III studie dat het vaccin CIMAvax-EGF wanneer gegeven in combinatie na vier chemokuren bijzonder extra goede resultaten geeft. Vooral bij patienten met een EGFR mutatie. En belangrijk om te weten dat ook andere vormen van kanker vaak een EGFR mutatie hebben, zoals tumoren van darmkanker, baarmoederhalskanker, eierstokkanker, prostaatkanker enz. Eigenlijk komt een EGFR mutatie voor bij bijna alle vormen van kanker met solide tumoren. (Tekst gaat verder onder afbeelding)
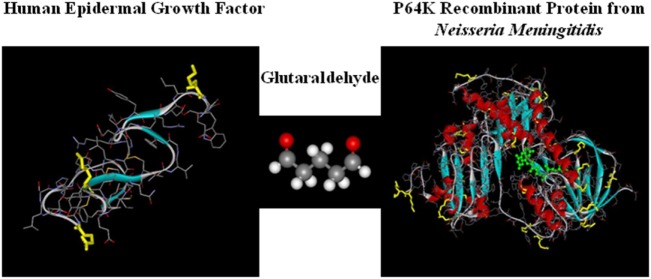
en deze reviewstudie vorige week gepubliceerd: CIMAvax-EGF: A New Therapeutic Vaccine for Advanced Non-Small Cell Lung Cancer Patients. Zie meer gegevens daarvan verderop in dit artikel.
Ook is er een nieuwe fase III studie (WHO-validated public registry; http://www.who.int/ictrp/network/rpcec/en, trial number RPCEC00000208) opengesteld waarin CIMAvax-EGF wordt gegeven als belangrijkste medicijn na vier chemokuren waarbij de EGF concentratie hoger is dan 870 pg/ml .
Table 2
CIMAvax-EGF in the treatment of patients with advanced NSCLC (Phase III clinical trial).
| Patient population | CIMAvax-EGF arm | Control arm | MST | |
|---|---|---|---|---|
| CIMAvax arm (months) | Control arm (months) | |||
| Stage IIIB/IV NSCLC patients, with at least stable disease after CTP (ITT) | CIMAvax-EGF | BSC | 10.83 | 8.86 |
| Stage IIIB/IV NSCLC patients, with at least stable disease after CTP (PP) | CIMAvax-EGF | BSC | 12.43 | 9.43 |
| Stage IIIB/IV NSCLC patients, with at least stable disease after CTP. Patients with (EGF) > 870 pg/ml | CIMAvax-EGF | BSC | 14.66 | 8.63 |
NSCLC, non-small cell lung cancer; MST, median survival time; PD, progressive disease; CTP, chemotherapy; BSC, best supportive care.
 CIMAvax werkingsmechanisme
CIMAvax werkingsmechanisme
Het vaccin wordt al 16 jaar onderzocht en heeft goede resultaten gegeven blijkt uit verschillende studies, zelfs bij patiënten met gevorderde uitgezaaide vormen van kanker. Het bijzondere aan dit vaccin is dat het weinig tot geen bijwerkingen geeft. Wat nog indrukwekkender is, is dat in een land als Cuba met weinig budget zo'n vaccin kan worden ontwikkeld zonder hulp van farmaceutische bedrijven.
Het vaccin werkt door een eiwit bekend als EGF (epidermale groeifactor) aan te vallen. EGF zorgt voor groei van longkankercellen maar ook van andere tumorcellen. Het CIMAvax vaccin versterkt het immuunsysteem en versnelt de productie van antilichamen die zich binden aan EGF, waardoor het kankercellen niet voedt en de ziekte zich niet verder kan verspreiden. Dit verbetert de levensduur van de patiënt en voorkomt de ziekte effectief. De toediening van het vaccin verlicht ook de symptomen van kanker en vermindert de pijn. Het vaccin wordt al gebruikt in landen zoals Bosnië en Herzegovina, Paraguay, Colombia en Peru.
Uit het studierapport: CIMAvax-EGF vaccine exerts its anti-cancer activity by targeting the immune system, inducing anti-EGF antibodies that result in the decline of the circulating EGF in sera (23, 24). This, in turn, significantly decreases the probability that the remaining EGF binds to its receptor (EGFR) on the surface of cancer cells. EGF withdrawal results in the loss of a key pro-proliferation and pro-survival signal for the neoplastic cells (23, 24). The vaccine has demonstrated to be safe and immunogenic in more than 5,000 advanced NSCLC patients (23, 24).
Cubaanse wetenschappers verwachten dat het vaccin borstkanker, baarmoederkanker en prostaattumoren kan genezen, terwijl er ook studies naar andere vormen van kanker lopen. Het vaccin is gratis voor Cubanen, terwijl mensen uit andere delen van de wereld wel moeten betalen maar wordt hun niet onthouden en is tegen betaling verkrijgbaar.
Voor onze donateurs hebben we de adresgegevens beschikbaar om dit vaccin te kopen of mee te doen aan een studie.
Deze reviewstudie is gratis in te zien: CIMAvax-EGF: A New Therapeutic Vaccine for Advanced Non-Small Cell Lung Cancer Patients.
Hier het abstract en referentielijst:
CIMAvax-EGF: A new therapeutic vaccine for advanced non-small cell lung cancer patients and other tumors with EGFR mutation (epidermal growth factor)
CIMAvax-EGF: A New Therapeutic Vaccine for Advanced Non-Small Cell Lung Cancer Patients
- Center of Molecular Immunology, Havana, Cuba
Lung cancer is the common fatal illness with the highest incidence and mortality globally. Epidermal growth factor receptor overexpression by tumor cells is associated with uncontrolled proliferation, angiogenesis, anti-apoptotic signals, metastization, and invasiveness. CIMAvax-EGF vaccine consists of a chemical conjugate of the EGF with the P64 protein derived from the Meningitis B bacteria and Montanide ISA 51, as adjuvant. The vaccine is projected to induce antibodies against EGF that results in EGF withdrawal. CIMAvax-EGF demonstrated to be safe and immunogenic in advanced non-small cell lung cancer (NSCLC) patients. The efficacy study was an open-label, multicentric Phase III clinical trial, which enrolled 405 advanced NSCLC patients. Patients with proven stage IIIB/IV NSCLC, who had completed four to six cycles of chemotherapy (CTP) were randomized to receive CIMAvax-EGF or best supportive care. CIMAvax-EGF resulted in a significantly larger overall survival in patients receiving at least four doses. High EGF concentration at baseline was a good predictive biomarker of the vaccine activity and a poor prognostic biomarker for the non-treated population. The proportion of CD8+CD28− cells, CD4 cells, and the CD4/CD8 ratio after first-line CTP was also associated with CIMAvax-EGF clinical benefit. After completing the Phase III, a Phase IV trial was done where the vaccine was administered in primary care units. Administering the vaccine at primary care institutions granted better access and treatment compliance. Safety was confirmed. Several clinical trials are currently ongoing to validate EGF as a predictive biomarker of CIMAvax-EGF efficacy.
CIMAvax-EGF in Primary Care Units and Future Perspectives
After completing the Phase III, a Phase IV trial was launched where the family medicine physicians administered CIMAvax-EGF in primary health care units (policlinics). In total, 45 primary level units together with 24 secondary level units (hospitals) participated in the study that enrolled more than 1,000 patients in 3 years. This study was registered in the National Public Registry of Clinical Trials (http://www.who.int/ictrp/network/rpcec/en, trial number RPCEC00000181). Administering the vaccine at primary care institutions granted better access and treatment compliance. Safety was confirmed; the most frequently reported adverse events were pain at the site of injection followed by fever, headache, chills, nausea, and dyspnea (22).
Overall survival of those patients that received at least one vaccine dose was 13.9 months (mean) and 7.0 months (median). Survival rate at 12 and 24 months was 34.8 of 18.1%, respectively. On the other hand, the overall survival of patients receiving at least the induction doses was 16.93 months (mean) and 9.9 months (median). The 12 and 24 months survival rate was of 44.1 and 23.3%, respectively.
In summary, CIMAvax-EGF was safe in patients with NSCLC at advanced stages treated in primary care facilities. The safety profile coincided with the previously described in controlled studies. CIMAvax-EGF also showed benefit in terms of survival, mainly in those subjects that completed four vaccine doses. Treatment with CIMAvax-EGF resulted in preliminary evidences of improvement in the quality of life, which was significant for the emotional functioning and the fatigue symptom. The use of medications to control pain was stable during vaccination (22).
Several clinical trials are currently ongoing. A new Phase III trial (WHO-validated public registry; http://www.who.int/ictrp/network/rpcec/en, trial number RPCEC00000208) is open for enrollment, where CIMAvax-EGF is used as switch maintenance in patients completing front-line CTP that has EGF concentration higher than 870 pg/ml (enrichment design). The main goal of the trial is to prospectively validate EGF as a predictive biomarker. In this scenario, the randomization is unbalanced (3:1) given the previous evidences of the clinical benefit of the vaccine. In addition, a new Phase IV (WHO-validated public registry; http://www.who.int/ictrp/network/rpcec/en, trial number PCEC00000205) was launched in 178 policlinics (at least one investigation site per state municipality) and 25 hospitals. Patients will be recruited by the oncologists in the specialized oncology services, but will be treated in their neighborhood, at the primary health care facilities. The aim is to grant vaccine access and to improve treatment compliance. In this trial, EGF concentration will be measured but not as an inclusion criterion. Instead, EGF at baseline will be retrospectively correlated with the clinical efficacy. An EGF quantification system was developed in the country by the National Center for Immunoassay, to accompany the vaccine prescription (37). Both studies will permit the consolidation of the scientific evidence of the EGF as a biomarker. Other translational studies are planned to gather more information on the relevance of the lymphocyte subpopulation as well as the individual tumor biology (mainly associated with EGFR mutations) for the CIMAvax-EGF efficacy.
Author Contributions
DS was involved in the evaluation of immunogenicity and predictive biomarkers of CIMAvax-EGF efficacy (EGF concentration and immunophenotyping). TC was involved in trials’ design and implementation. Both authors participated in the analysis, writing, and revision of the manuscript.
Conflict of Interest Statement
Both authors, DS and TC, are employees of the Center of Molecular Immunology, the institution that owns the patent and manufactures CIMAvax-EGF. Neither author receive additional compensation associated with CIMAvax-EGF registration or marketing.
Acknowledgments
Both authors are very grateful to the research teams from the hospitals or the primary health care institutions participating in the study. Their contribution to the project has been invaluable, for 20 years. The authors are also extremely thankful to our patients and their relatives that supported unconditionally the clinical research.
Funding
This research was funded by the Center of Molecular Immunology and the National Ministry of Health.
the CIMAvax-EGF vaccine induces an EGF-specific protective humoral response in a high percent of NSCLC vaccinated patients, the quantity and quality of which were associated with clinical benefit
CIMAvaxEGF vaccine is a safe treatment option for advanced NSCLC that can be safely administered at primary level of health care. The median OS of treated patients (unselected population) compares with the results reported for second–line treatments and switch maintenance therapies. Patients who completed the induction phase of the treatment reached a better OS
Safety and efficacy of CIMAvax-EGF vaccine for the treatment of real-world non-small cell lung cancer patients
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
Department of Clinical Trials, Center of Molecular Immunology, 216 St and 15 Ave., Atabey, Playa, Havana 11600 Cuba
DOI: 10.15761/ICM.1000195
Abstract
Objectives: CIMAvax-EGF is a therapeutic vaccine registered as switch maintenance therapy. This vaccine induces antibodies against self EGF that affect EGF-EGFR interaction. The aim of this study was to evaluate safety and efficacy of CIMAvax-EGF in the context of primary care.
Methods: A phase IV clinical trial was conducted in 65 Policlinic areas and 16 hospitals in Cuba during 3 years. A total of 1081 advanced NSCLC patients were included without other treatment options due to progressive disease or comorbidities. CIMAvax-EGF was administered by intramuscular injection in four sites of administration (4 subdoses of 0.25 ml), every 2 weeks the first 4 doses and after this induction phase monthly reinmunizations were given.
Results: A total of 927 patients (85.7 %) received at least one dose of CIMAvaxEGF. Most frequently adverse events related to vaccine were: injection-site reaction (14.5%), fever (7.0%), headache (5.8%), tremors (4.3%), and nausea (4.3%). Most of them were grade1 -2 according CTCAE v3. There were no deaths related to CIMAvaxEGF. The median overall survival (mOS) time for all vaccinated patients was 7.0 months, and in a subgroup of patient who received at least 4 doses of CIMAvaxEGF mOS was 9.98 months (n=715). Patients treated as switch maintenance therapy (n=97) reached a mOS of 12.1 months. In a subgroup of unfit patients (n=213) mOS was 3.97 months, but in those who completed the induction phase mOS was 7.36 months (n=124). Emotional function was improved at months 6 and 12 compared to baseline.
Conclusions: CIMAvaxEGF vaccine is a safe treatment option for advanced NSCLC that can be safely administered at primary level of health care. The median OS of treated patients (unselected population) compares with the results reported for second–line treatments and switch maintenance therapies. Patients who completed the induction phase of the treatment reached a better OS.
Discussion
Results from this study confirm the safety profile of CIMAvax-EGF in the context of primary care assistance. The frequency of AE described here is consistent with previous clinical studies with the vaccine at secondary level of health care. Most part of vaccine-related adverse events was mild or moderate. A very low percentage of patients in these setting of population presented a serious AE related to CIMAvax-EGF and there was no death related.
In terms of efficacy the median overall survival in vaccinated patients compares with reported data from second-line drug studies. The median overall survival in our study (6.0 m) is similar to docetaxel (7.5 m), erlotinib (6.7 m) and pemetrexed (8.3 m), in unselected population [3-5]. Also, it is inferior to other results in this scenario with checkpoint inhibitors (nivolumab: 12.2 m non-squamous NSCLC, 9.2 m squamous NSCLC; atezolizumab: 12.6 m; pembrolizumab: 14.9 m) [6-9]. The lack of other lines of therapy in our population and the presence of 213 patients unfit for chemotherapy could be the reasons for this minor median OS.
The group of patients treated as switch maintenance therapy reached a median OS of 12.1 months (ITT population). In this scenario the efficacy of CIMAvax-EGF compares with other drugs registered: docetaxel (12.3 months), pemetrexed (13.4 m) and erlotinib (12.0 m) [10-12]. This result is also consistent with median overall survival reported in the phase III trial of CIMAvax-EGF (10.83 m).
As in previous trial with CIMAvax-EGF vaccine those patients who completed the induction phase of the treatment obtained a benefit in terms of overall survival. It has been observed in our study in different settings: second-line, switch maintenance and unfit patients. It should be noted the existence of a tail in the OS curves of those populations that reflects the minor probability of patient’s death in that period.
Quality of life data was evaluable only in 25.1 % (n=234) of patients treated with CIMAvax-EGF. There was a significant difference between baseline and post-treatment evaluations at month 6 and 12 in emotional function and fatigue symptoms. Also, most of the QLQ-LC 13 symptoms were significant different at month 6 vs baseline. The type of the study and the small number of patients at each evaluation could affect the interpretation of these data.
In conclusion, CIMAvax-EGF is an effective and safe treatment option for advanced NSCLC patients treated at primary level of health care. Also, this vaccine can be administered for a long-term period without cumulative toxicity due to its favorable safety profile. The completion of the induction phase is a critical point for developing a protective response ensuring a clinical stabilization of the disease.
Funding
The trial was funded by Center of Molecular Immunology and Cuban Ministry of Public Health.
Acknowledgments
We thank all the participating patients and their families, staffs of all the primary care units and hospitals involved, and National Coordinating Center for Clinical Trials as the CRO of the study.
References
- 1. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, et al. (2014) SEER Cancer Statistics Review, 1975- 2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017.
- 2. Rodriguez PC, Popa X, Martinez O (2016) A Phase III Clinical Trial of the Epidermal Growth Factor Vaccine CIMAvaxEGF as Switch Maintenance Therapy in Advanced Non-Small Cell Lung Cancer Patients. Clin Cancer Res 22: 3782-3790.
- 3. Shepherd FA, Dancey J, Ramlau R, Gralla R, O'Rourke M, et al. (2000) Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18: 2095-03.
- 4. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, et al. (2005) Erlotinib in Previously Treated Non–Small Cell Lung Cancer. N Eng J Med 353: 123-132.
- 5. Hanna N, Shepherd FA, Fossella FV, Pereira JR, Marinis FD, et al. (2004) Randomized Phase III Trial of Pemetrexed versus Docetaxel in Patients with Non–Small-Cell Lung Cancer Previously Treated with Chemotherapy. J Clin Oncol 22: 1589-97.
- 6. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, et al. (2015) Nivolumab versus Docetaxel in Advanced Non-Squamous Non-Small Cell Lung Cancer. N Eng J Med 373: 1627-1639.
- 7. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, et al. (2015) Nivolumab versus Docetaxel in Advanced Squamous Cell Non-Small Cell Lung Cancer. N Eng J Med 373: 123-135.
- 8. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, et al. (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 387: 1837-1846.
- 9. Herbst RS, Baas P, Kim D, Felip E, Pérez-Gracia JL, et al. (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1- positive, advanced non-small-cell lung cancer (KEYNOTE-010): A Randomised controlled trial. Lancet 387: 1540-1550.
- 10. Fidias PM, Dakhil SR, Lyss AP, Loesch DM, Waterhouse DM, et al. (2008) Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 27: 591-598.
- 11. Ciuleanu T, Brodowicz T, Zielinski C, Kim JH, Krzakowski M, et al. (2009) Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double blind, phase 3 study. Lancet 374: 1432-1440.
- 12. Capuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczésna A, et al. (2010) Erlotinib as maintenance treatment in advanced non small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 11: 521-529.
References
Gerelateerde artikelen
- Longkanker: CIMAvax-EGF vaccin geeft uitstekende resultaten op overall overleving en ziektevrije tijd bij longkanker en andere vormen van kanker met EGFR mutatie
- Longkanker: Specifieke bloedwaarden en biomarkers die wijzen op veroudering van immuunsysteem plus basale EGF-concentratie in bloed resulteerden in goede voorspellers van het succes van het CIMAvax-EGF vaccin bij gevorderde longkanker
- Longkanker: Peptide vaccin - L-BLP25 - tegen uitgezaaide inoperabele niet-klein-cellige longkanker - fase IIIB - effectief. Nieuwe fase III studie moet resultaten bevestigen
- Longkanker: Succes van vaccin tegen longkanker (ook in Leuven /Brussel) wordt alleen bereikt met hulp van Mage=A3 eiwit tegen terugkeer van operatief verwijderde niet-klein-cellige longkanker. Artikelupdate 14 juni 2008
- Longkanker: Immuuntherapie met Nivolumab + chemotherapie geeft op 3-jaars meting bij patienten met operabele niet-kleincellige longkanker (NSCLC) betere ziektevrije overleving (57 procent vs 43 procent) in vergelijking met alleen chemotherapie



 Danay Saavedra
Danay Saavedra Tania Crombet
Tania Crombet
Plaats een reactie ...
1 Reactie op "Longkanker: CIMAvax-EGF vaccin geeft uitstekende resultaten op overall overleving en ziektevrije tijd bij longkanker en andere vormen van kanker met EGFR mutatie"