22 april 2022: Bron: BMC Cancer volume 20, Article number: 772 (2020)
Eerder al hebben we informatie over het Cubaanse CIMAvax-EGF vaccin bij niet-kleincellige longkanker gegeven in dit artikel. N.a.v. een vraag van een bezoeker onderaan dat artikel ben ik op zoek gegaan naar extra informatie en kwam daar paar interessante nieuwe studies tegen. Dit CIMAvax-EGF vaccin zou ook wel eens effectief kunnen zijn bij andere vormen van kanker met solide tumoren met een EGFR mutatie. De epidermale groeifactorreceptor (EGFR) is een signaaleiwit dat zich op de celmembraan bevindt. Wanneer de epidermale groeifactor (EGF) zich bindt aan deze receptor, dan leidt dit tot celgroei en celdeling.
CIMAvax-EGF bleek eerder al veilig en effectief te zijn bij de behandeling van patiënten met gevorderde niet-kleincellige longkanker (NSCLC) in verschillende klinische onderzoeken [zie referenties 1,2,3,4,5]. Er zijn echter aanwijzingen voor verschillende reacties op het vaccin. De ene patiént reageert wel goed, de andere patiënt helemaal niet. In deze studie [zie ref. 6] werden patiënten met korte- en langetermijnoverleving onderscheiden tussen degenen die werden behandeld met CIMAvax-EGF.
In verschillende fase II- en fase IIL studies die afgelopen jaren werden uitgevoerd, blijkt de patiënt die een "goede antilichaamrespons" (anti-EGF-antilichaamtiters ≥ 1:4000 seraverdunning) ontwikkelde een beduidend betere overleving te hebben in vergelijking met patiënten die een lagere anti-EGF-antilichaamrespons hadden. [zie referenties 1, 3, 4].
Aan de andere kant werd de correlatie tussen EGF-concentratie bij aanvang van de behandeling en uiteindelijke overlevingsduur waargenomen sinds de eerste fase I-studie [zie referentie 5]. De daaropvolgende studies bevestigden ook dit feit, gevaccineerde patiënten met een serum basale EGF-concentratie > 870 pg/ml vertoonden een betere mediane overleving in vergelijking met controles met hetzelfde EGF-serumniveau [1, 2].
Bovendien waren biomarkers die wijzen op veroudering van het immuunsysteem (immunosenescentie-biomarkers) als deel uitmakend van CD8 + CD28−-cellen, CD4-cellen en de CD4/CD8-ratio na eerstelijns chemotherapie ook geassocieerd met klinisch voordeel van het CIMAvax-EGF vaccin. Al deze onderzoeken wijzen op het belang dat wordt gehecht aan het zoeken naar voorspellende biomarkers die het mogelijk maken om patiënten te selecteren die echt voordeel kunnen halen uit het vaccin.
En die voorspellende biomarkers zijn nu gezamenlijk en apart onderzocht. Zo blijkt uit deze studie (abstract staat verderop in dit artikel) dat bepaalde bloedwaarden (perifere bloedparameters) en biomarkers die wijzen op veroudering van het immuunsysteem (immunosenescentie-biomarkers) samen met een basale EGF-concentratie in het bloed goede voorspellers van het wel of niet mogelijke aanslaan van het CIMAvax-EGF vaccin bij patiënten met gevorderde niet-kleincellige longkanker.
Zo werden de gegevens van patiënten uit een gecontroleerde klinische studie retrospectief geanalyseerd om te kijken welke biomarkers voor het effect van het CIMAvax-EGF vaccin voorspellende waarde hadden.
Mogelijke voorspellende biomarkers voor de behandeling die werden onderzocht waren basale serum EGF-concentratie, perifere bloedparameters en immunosenescentie-biomarkers. Met daarbij het aandeel dat CD8 + CD28- T-cellen, CD4+ en CD8+ T-cellen, CD4/CD8-verhouding en CD19+ B-cellen daarin hadden.
In de analyse werden ook de 33 patiënten die goed reageerden op het CIMAvax-EGF vaccin meegenomen in de analyse, maar dus ook de patiënten die minder goed reageerden of helemaal niet reageerden. Voor alle mogelijke modellen is de voorspellende causale informatie (PCI) berekend. Het model met een minimaal aantal voorspellers, maar met een hoge voorspellingsnauwkeurigheid (PCI > 0.7) werd geselecteerd.
Uit de analyse bleek dat het gemiddelde van de voorspellende causale informatie (PCI) steeg van 0,486, wanneer slechts één voorspeller werd bekeken, tot 0,98 bij gebruik van de multivariate benadering met alle voorspellers.
Het model dat het aantal CD4+ T-cellen, basale epidermale groeifactor (EGF)-concentratie, neutrofiel tot lymfocytverhouding, monocyten en neutrofielen als voorspellers beschouwt, werd geselecteerd (PCI > 0.74).
Patiënten die volgens de biomarkerwaarden vóór de behandeling werden voorspeld als goede responders die met CIMAvax-EGF werden behandeld, hadden een significant hogere waargenomen overleving vergeleken met de controlegroep (p = 0,03). Er werd geen verschil waargenomen voor slechte responders tussen de prognose en het uiteindelijke resultaat.
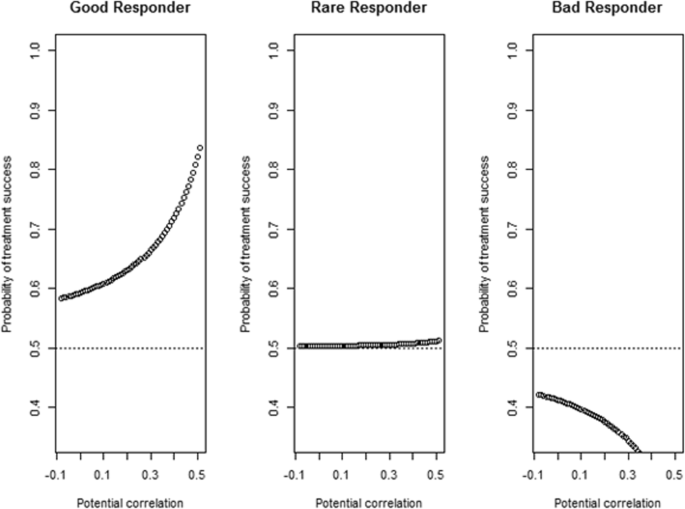
Predictive probability of treatment success for three examples of a Good responder (basal EGF concentration = 1700, CD4+ T cells = 65, CD4/CD8 ratio = 3, NLR = 2, Neutrophils = 50), b Rare (basal EGF concentration = 900, CD4+ T cells =35, CD4/CD8 ratio = 3, NLR = 2, Neutrophils = 55) and c Bad responders (basal EGF concentration = 200, CD4+ T cells =10, CD4/CD8 ratio = 1, NLR = 1, Neutrophils = 60) to CIMAvax-EGF
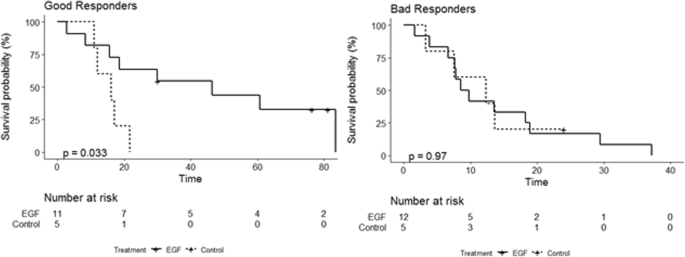
Kaplan Meier survival curves for patient treated with CIMAvax-EGF and control for a) good responders, b) bad responders
Klein stukje nog uit Wikipedia over CIMAvax-EGF vaccin met daarin verwijzingen naar studies die al gedaan zijn en nog lopende studies, ook in Europa verkrijgbaar:
CimaVax-EGF is a vaccine used to treat cancer, specifically non-small-cell lung carcinoma (NSCLC). CIMAvax-EGF is composed of recombinant human epidermal growth factor (EGF) conjugated to a protein carrier.[1]
The vaccine was developed by the Center of Molecular Immunology, Havana, Cuba.[2][3] There are agreements in place to test it in the United States, Japan, and some European countries.[4] It is currently available in Cuba, Colombia, Bosnia and Herzegovina, Peru and Paraguay.[5] In October 2015 Serbia's Institute of Virology, Vaccines and Sera (AKA Torlak Institute) signed a memorandum for use in 30 patients as part of a study.[6] CimaVax is relatively cheap to produce and store, and has low toxicity.[4] Side effects of the vaccine appear to be mild, and include chills, fever, and feeling sick.[7][8]>>>>>>lees verder
Hier de twee abstracten van bovengenoemde studies met bijbehorende referenties. In deze eerste studie ook verwijzingen naar CIMAvax-EGF gegeven in combinatie met anti-PD medicijnen, met ook studies bij andere vormen van kanker waaronder darmkanker, alvleesklierkanker enz. maar lees het hele studierapport want is teveel om dat hier apart te noemen. Klik op de titel van het abstract:
ABSTRACT
We previously reported that CIMAvax-EGF vaccine is safe, immunogenic and efficacious to treat advanced non-small-cell lung cancer (NSCLC) patients. A phase III trial was designed using an optimized immunization schedule. It included higher antigen dose and injections at multiple sites. Immune response and circulating biomarkers were studied in a subset of patients. EGF-specific antibody titers, IgG subclasses, peptide immunodominance and circulating biomarkers were assessed by ELISA. In vitro EGF-neutralization capacity of immune sera and EGF-IgG binding kinetics was evaluated by Western Blot and Surface Plasmon Resonance (SPR) technology, respectively. We show that CIMAvax-EGF elicited mainly IgG3/IgG4 antibodies at titers exceeding 1:4000 in 80% of vaccinated patients after 3 months of treatment. The EGF-specific humoral response was directed against the central region of the EGF molecule. For the first time, the kinetic constants of EGF-specific antibodies were measured evidencing affinity maturation of antibody repertoire up to month 12 of vaccination. Notably, the capacity of post-immune sera to inhibit EGFR phosphorylation significantly increased during the course of the immunization scheme and was related to clinical outcome (P = .013, log-rank test). Basal concentrations of EGF and TGFα in the serum were affected by EGF-based immunization. In conclusion, the CIMAvax-EGF vaccine induces an EGF-specific protective humoral response in a high percent of NSCLC vaccinated patients, the quantity and quality of which were associated with clinical benefit (clinical trial registration number: RPCEC00000161, http://registroclinico.sld.cu/).
- Research article
- Open Access
- Published:
Identifying predictive biomarkers of CIMAvaxEGF success in non–small cell lung cancer patients
BMC Cancer volume 20, Article number: 772 (2020)
Abstract
Background
Immunosenescence biomarkers and peripheral blood parameters are evaluated separately as possible predictive markers of immunotherapy. Here, we illustrate the use of a causal inference model to identify predictive biomarkers of CIMAvaxEGF success in the treatment of Non–Small Cell Lung Cancer Patients.
Methods
Data from a controlled clinical trial evaluating the effect of CIMAvax-EGF were analyzed retrospectively, following a causal inference approach. Pre-treatment potential predictive biomarkers included basal serum EGF concentration, peripheral blood parameters and immunosenescence biomarkers. The proportion of CD8 + CD28- T cells, CD4+ and CD8+ T cells, CD4/CD8 ratio and CD19+ B cells. The 33 patients with complete information were included. The predictive causal information (PCI) was calculated for all possible models. The model with a minimum number of predictors, but with high prediction accuracy (PCI > 0.7) was selected. Good, rare and poor responder patients were identified using the predictive probability of treatment success.
Results
The mean of PCI increased from 0.486, when only one predictor is considered, to 0.98 using the multivariate approach with all predictors. The model considering the proportion of CD4+ T cell, basal Epidermal Growth Factor (EGF) concentration, neutrophil to lymphocyte ratio, Monocytes, and Neutrophils as predictors were selected (PCI > 0.74). Patients predicted as good responders according to the pre-treatment biomarkers values treated with CIMAvax-EGF had a significant higher observed survival compared with the control group (p = 0.03). No difference was observed for bad responders.
Conclusions
Peripheral blood parameters and immunosenescence biomarkers together with basal EGF concentration in serum resulted in good predictors of the CIMAvax-EGF success in advanced NSCLC. Future research should explore molecular and genetic profile as biomarkers for CIMAvax-EGF and it combination with immune-checkpoint inhibitors. The study illustrates the application of a new methodology, based on causal inference, to evaluate multivariate pre-treatment predictors. The multivariate approach allows realistic predictions of the clinical benefit of patients and should be introduced in daily clinical practice.
References
- Global Burden of Disease Cancer C. Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019. doi:https://doi.org/10.1001/jamaoncol.2019.2996. , [Web of Science ®], [Google Scholar]
- Jemal A. Global burden of cancer: opportunities for prevention. Lancet. 2012;380:1797–11. doi:https://doi.org/10.1016/S0140-6736(12)61688-2. , , [Web of Science ®], [Google Scholar]
- Gridelli C, Maione P, Rossi A, Guerriero C, Ferrara C, Del Gaizo F, Colantuoni G, Nicolella D, Napolitano L. Chemotherapy of advanced NSCLC in special patient population. Ann Oncol. 2006;17(Suppl 5):v72–78. doi:https://doi.org/10.1093/annonc/mdj955. , , [Google Scholar]
- Langer CJ. Emerging immunotherapies in the treatment of non-small cell lung cancer (NSCLC): the role of immune checkpoint inhibitors. Am J Clin Oncol. 2015;38:422–430. doi:https://doi.org/10.1097/COC.0000000000000059. , , [Web of Science ®], [Google Scholar]
- Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi:https://doi.org/10.1016/1040-8428(94)00144-I. , , [Web of Science ®], [Google Scholar]
- Ryan PD, Chabner BA. On receptor inhibitors and chemotherapy. Clin Cancer Res. 2000;6:4607–4609. , [Web of Science ®], [Google Scholar]
- Prigent SA, Lemoine NR. The type 1 (EGFR-related) family of growth factor receptors and their ligands. Prog Growth Factor Res. 1992;4:1–24. doi:https://doi.org/10.1016/0955-2235(92)90002-Y. , , [Google Scholar]
- Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer. 2001;8:11–31. doi:https://doi.org/10.1677/erc.0.0080011. , , [Web of Science ®], [Google Scholar]
- Tominaga T, Tsuchiya T, Mochinaga K, Arai J, Yamasaki N, Matsumoto K, Miyazaki T, Nagasaki T, Nanashima A, Tsukamoto K, et al. Epidermal growth factor signals regulate dihydropyrimidine dehydrogenase expression in EGFR-mutated non-small-cell lung cancer. BMC Cancer. 2016;16:354. doi:https://doi.org/10.1186/s12885-016-2392-0. , , [Web of Science ®], [Google Scholar]
- Yonemura Y, Takamura H, Ninomiya I, Fushida S, Tsugawa K, Kaji M, Nakai Y, Ohoyama S, Yamaguchi A, Miyazaki I. Interrelationship between transforming growth factor-alpha and epidermal growth factor receptor in advanced gastric cancer. Oncology. 1992;49:157–161. doi:https://doi.org/10.1159/000227031. , , [Web of Science ®], [Google Scholar]
- Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, Gondi V, Hsu KT, Harari PM. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi:https://doi.org/10.1038/onc.2008.19. , , [Web of Science ®], [Google Scholar]
- Ishikawa N, Daigo Y, Takano A, Taniwaki M, Kato T, Hayama S, Murakami H, Takeshima Y, Inai K, Nishimura H, et al. Increases of amphiregulin and transforming growth factor-alpha in serum as predictors of poor response to gefitinib among patients with advanced non-small cell lung cancers. Cancer Res. 2005;65:9176–9184. doi:https://doi.org/10.1158/0008-5472.CAN-05-1556. , , [Web of Science ®], [Google Scholar]
- Fontanini G, De Laurentiis M, Vignati S, Chine S, Lucchi M, Silvestri V, Mussi A, De Placido S, Tortora G, Bianco AR, et al. Evaluation of epidermal growth factor-related growth factors and receptors and of neoangiogenesis in completely resected stage I-IIIA non-small-cell lung cancer: amphiregulin and microvessel count are independent prognostic indicators of survival. Clin Cancer Res. 1998;4:241–249. , [Web of Science ®], [Google Scholar]
- Gonzalez G, Crombet T, Torres F, Catala M, Alfonso L, Osorio M, Neninger E, Garcia B, Mulet A, Perez R, et al. Epidermal growth factor-based cancer vaccine for non-small-cell lung cancer therapy. Ann Oncol. 2003;14:461–466. doi:https://doi.org/10.1093/annonc/mdg102. , , [Web of Science ®], [Google Scholar]
- Ramos TC, Vinageras EN, Ferrer MC, Verdecia BG, Rupale IL, Perez LM, Marinello GG, Rodriguez RP, Davila AL. Treatment of NSCLC patients with an EGF-based cancer vaccine: report of a phase I trial. Cancer Biol Ther. 2006;5:145–149. doi:https://doi.org/10.4161/cbt.5.2.2334. [Taylor & Francis Online], [Web of Science ®], [Google Scholar]
- Neninger E, Verdecia BG, Crombet T, Viada C, Pereda S, Leonard I, Mazorra Z, Fleites G, Gonzalez M, Wilkinson B, et al. Combining an EGF-based cancer vaccine with chemotherapy in advanced nonsmall cell lung cancer. J Immunother. 2009;32:92–99. doi:https://doi.org/10.1097/CJI.0b013e31818fe167. , , [Web of Science ®], [Google Scholar]
- Garcia B, Neninger E, de la Torre A, Leonard I, Martinez R, Viada C, Gonzalez G, Mazorra Z, Lage A, Crombet T. Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti-epidermal growth factor antibodies is related to better survival in advanced non-small-cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clin Cancer Res. 2008;14:840–846. doi:https://doi.org/10.1158/1078-0432.CCR-07-1050. , , [Web of Science ®], [Google Scholar]
- Rodriguez PC, Neninger E, Garcia B, Popa X, Viada C, Luaces P, Gonzalez G, Lage A, Montero E, Crombet T. Safety, immunogenicity and preliminary efficacy of multiple-site vaccination with an Epidermal Growth Factor (EGF) based cancer vaccine in advanced non small cell lung cancer (NSCLC) patients. J Immune Based Ther Vaccines. 2011;9:7. doi:https://doi.org/10.1186/1476-8518-9-7. , , [Google Scholar]
- Rodriguez PC, Popa X, Martinez O, Mendoza S, Santiesteban E, Crespo T, Amador RM, Fleytas R, Acosta SC, Otero Y, et al. A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2016;22:3782–3790. doi:https://doi.org/10.1158/1078-0432.CCR-15-0855. , , [Web of Science ®], [Google Scholar]
- Santana H, Garcia G, Vega M, Beldarrain A, Paez R. Stability studies of a freeze-dried recombinant human epidermal growth factor formulation for wound healing. PDA J Pharm Sci Technol. 2015;69:399–416. doi:https://doi.org/10.5731/pdajpst.2015.01052. , , [Google Scholar]
- Hahnefeld C, Drewianka S, Herberg FW. Determination of kinetic data using surface plasmon resonance biosensors. Methods Mol Med. 2004;94:299–320. doi:https://doi.org/10.1385/1-59259-679-7:299. , [Google Scholar]
- Pol E, Karlsson R, Roos H, Jansson A, Xu B, Larsson A, Jarhede T, Franklin G, Fuentes A, Persson S. Biosensor-based characterization of serum antibodies during development of an anti-IgE immunotherapeutic against allergy and asthma. J Mol Recognit. 2007;20:22–31. doi:https://doi.org/10.1002/jmr.804. , , [Web of Science ®], [Google Scholar]
- Klasse PJ. How to assess the binding strength of antibodies elicited by vaccination against HIV and other viruses. Expert Rev Vaccines. 2016;15:295–311. doi:https://doi.org/10.1586/14760584.2016.1128831. [Taylor & Francis Online], [Web of Science ®], [Google Scholar]
- Poulsen TR, Jensen A, Haurum JS, Andersen PS. Limits for antibody affinity maturation and repertoire diversification in hypervaccinated humans. J Immunol. 2011;187:4229–4235. doi:https://doi.org/10.4049/jimmunol.1000928. , , [Web of Science ®], [Google Scholar]
- Yasmeen A, Ringe R, Derking R, Cupo A, Julien JP, Burton DR, Ward AB, Wilson IA, Sanders RW, Moore JP, et al. Differential binding of neutralizing and non-neutralizing antibodies to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits. Retrovirology. 2014;11:41. doi:https://doi.org/10.1186/1742-4690-11-41. , , [Web of Science ®], [Google Scholar]
- Mutsaers AJ, Francia G, Man S, Lee CR, Ebos JM, Wu Y, Witte L, Berry S, Moore M, Kerbel RS. Dose-dependent increases in circulating TGF-alpha and other EGFR ligands act as pharmacodynamic markers for optimal biological dosing of cetuximab and are tumor independent. Clin Cancer Res. 2009;15:2397–2405. doi:https://doi.org/10.1158/1078-0432.CCR-08-1627. , , [Web of Science ®], [Google Scholar]
- Loupakis F, Cremolini C, Fioravanti A, Orlandi P, Salvatore L, Masi G, Schirripa M, Di Desidero T, Antoniotti C, Canu B, et al. EGFR ligands as pharmacodynamic biomarkers in metastatic colorectal cancer patients treated with cetuximab and irinotecan. Target Oncol. 2014;9:205–214. doi:https://doi.org/10.1007/s11523-013-0284-7. , , [Web of Science ®], [Google Scholar]
- Rodriguez PC, Gonzalez I, Gonzalez A, Avellanet J, Lopez A, Perez R, Lage A, Montero E. Priming and boosting determinants on the antibody response to an Epidermal Growth Factor-based cancer vaccine. Vaccine. 2008;26:4647–4654. doi:https://doi.org/10.1016/j.vaccine.2008.07.003. , , [Web of Science ®], [Google Scholar]
- Saavedra D, Crombet T. CIMAvax-EGF: a new therapeutic vaccine for advanced non-small cell lung cancer patients. Front Immunol. 2017;8:269. doi:https://doi.org/10.3389/fimmu.2017.00269. , , [Web of Science ®], [Google Scholar]
- Mould RC, AWK A, van Vloten JP, Susta L, Mutsaers AJ, Petrik JJ, Wood GA, Wootton SK, Karimi K, Bridle BW. Enhancing immune responses to cancer vaccines using multi-site injections. Sci Rep. 2017;7:8322. doi:https://doi.org/10.1038/s41598-017-08665-9. , , [Web of Science ®], [Google Scholar]
- Jaffee EM, Thomas MC, Huang AY, Hauda KM, Levitsky HI, Pardoll DM. Enhanced immune priming with spatial distribution of paracrine cytokine vaccines. J Immunother Emphasis Tumor Immunol. 1996;19:176–183. doi:https://doi.org/10.1097/00002371-199605000-00002. , , [Web of Science ®], [Google Scholar]
- Kim A, Sadegh-Nasseri S. Determinants of immunodominance for CD4 T cells. Curr Opin Immunol. 2015;34:9–15. doi:https://doi.org/10.1016/j.coi.2014.12.005. , , [Web of Science ®], [Google Scholar]
- Kyogoku N, Ikeda H, Tsuchikawa T, Abiko T, Fujiwara A, Maki T, Yamamura Y, Ichinokawa M, Tanaka K, Imai N, et al. Time-dependent transition of the immunoglobulin G subclass and immunoglobulin E response in cancer patients vaccinated with cholesteryl pullulan-melanoma antigen gene-A4 nanogel. Oncol Lett. 2016;12:4493–4504. doi:https://doi.org/10.3892/ol.2016.5253. , , [Web of Science ®], [Google Scholar]
- Valenzuela NM, Schaub S. The biology of IgG subclasses and their clinical relevance to transplantation. Transplantation. 2018;102:S7–S13. doi:https://doi.org/10.1097/TP.0000000000001816. , , [Web of Science ®], [Google Scholar]
- Carpenter G, Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi:https://doi.org/10.1146/annurev.bi.48.070179.001205. , , [Web of Science ®], [Google Scholar]
- Ullenhag GJ, Frodin JE, Strigard K, Mellstedt H, Magnusson CG. Induction of IgG subclass responses in colorectal carcinoma patients vaccinated with recombinant carcinoembryonic antigen. Cancer Res. 2002;62:1364–1369. , [Web of Science ®], [Google Scholar]
- Oji A, Noda T, Fujihara Y, Miyata H, Kim YJ, Muto M, Nozawa K, Matsumura T, Isotani A, Ikawa M. CRISPR/Cas9 mediated genome editing in ES cells and its application for chimeric analysis in mice. Sci Rep. 2016;6:31666. doi:https://doi.org/10.1038/srep31666. , , [Web of Science ®], [Google Scholar]
- Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy. 2009;39:469–477. doi:https://doi.org/10.1111/j.1365-2222.2009.03207.x. , , [Web of Science ®], [Google Scholar]
- Senti G, Von Moos S, Tay F, Graf N, Sonderegger TJP, Kundig TM. Epicutaneous allergen-specific immunotherapy ameliorates grass pollen-induced rhinoconjunctivitis: A double-blind, placebo-controlled dose escalation study. J Allergy Clin Immunol. 2012;129:128–135. doi:https://doi.org/10.1016/j.jaci.2011.08.036. , , [Web of Science ®], [Google Scholar]
- Deisenhammer F, Reindl M, Berger T. Immunoglobulin subclasses in patients with neutralizing and nonneutralizing antibodies against IFN-beta1b. J Interferon Cytokine Res. 2001;21:167–171. doi:https://doi.org/10.1089/107999001750133195. , , [Web of Science ®], [Google Scholar]
- Morera Y, Sanchez J, Bequet-Romero M, Selman-Housein KH, de la Torre A, Hernandez-Bernal F, Martin Y, Garabito A, Pinero J, Bermudez C, et al. Specific humoral and cellular immune responses in cancer patients undergoing chronic immunization with a VEGF-based therapeutic vaccine. Vaccine. 2017;35:3582–3590. doi:https://doi.org/10.1016/j.vaccine.2017.05.020. , , [Web of Science ®], [Google Scholar]
- Collins AM, Jackson KJ. A temporal model of human IgE and IgG antibody function. Front Immunol. 2013;4:235. doi:https://doi.org/10.3389/fimmu.2013.00235. , , [Web of Science ®], [Google Scholar]
- Esparis-Ogando A, Montero JC, Arribas J, Ocana A, Pandiella A. Targeting the EGF/HER ligand-receptor system in cancer. Curr Pharm Des. 2016;22:5887–5898. doi:https://doi.org/10.2174/1381612822666160715132233. , , [Web of Science ®], [Google Scholar]
- Taniguchi H, Takeuchi S, Fukuda K, Nakagawa T, Arai S, Nanjo S, Yamada T, Yamaguchi H, Mukae H, Yano S. Amphiregulin triggered epidermal growth factor receptor activation confers in vivo crizotinib-resistance of EML4-ALK lung cancer and circumvention by epidermal growth factor receptor inhibitors. Cancer Sci. 2017;108:53–60. doi:https://doi.org/10.1111/cas.13111. , , [Web of Science ®], [Google Scholar]
- Pentheroudakis G, Kotoula V, De Roock W, Kouvatseas G, Papakostas P, Makatsoris T, Papamichael D, Xanthakis I, Sgouros J, Televantou D, et al. Biomarkers of benefit from cetuximab-based therapy in metastatic colorectal cancer: interaction of EGFR ligand expression with RAS/RAF, PIK3CA genotypes. BMC Cancer. 2013;13:49. doi:https://doi.org/10.1186/1471-2407-13-49. , , [Web of Science ®], [Google Scholar]
- Yoshida M, Shimura T, Sato M, Ebi M, Nakazawa T, Takeyama H, Joh T. A novel predictive strategy by immunohistochemical analysis of four EGFR ligands in metastatic colorectal cancer treated with anti-EGFR antibodies. J Cancer Res Clin Oncol. 2013;139:367–378. doi:https://doi.org/10.1007/s00432-012-1340-x. , , [Web of Science ®], [Google Scholar]
- Gonzalez G, Crombet T, Neninger E, Viada C, Lage A. Therapeutic vaccination with epidermal growth factor (EGF) in advanced lung cancer: analysis of pooled data from three clinical trials. Hum Vaccin. 2007;3:8–13. doi:https://doi.org/10.4161/hv.3.1.3537. [Taylor & Francis Online], [Web of Science ®], [Google Scholar]
- Oji Y, Hashimoto N, Tsuboi A, Murakami Y, Iwai M, Kagawa N, Chiba Y, Izumoto S, Elisseeva O, Ichinohasama R, et al. Association of WT1 IgG antibody against WT1 peptide with prolonged survival in glioblastoma multiforme patients vaccinated with WT1 peptide. Int J Cancer. 2016;139:1391–1401. doi:https://doi.org/10.1002/ijc.30182. , , [Web of Science ®], [Google Scholar]
- Hansen GL, Gaudernack G, Brunsvig PF, Cvancarova M, Kyte JA. Immunological factors influencing clinical outcome in lung cancer patients after telomerase peptide vaccination. Cancer Immunol Immunother. 2015;64:1609–1621. doi:https://doi.org/10.1007/s00262-015-1766-5. , , [Web of Science ®], [Google Scholar]
References
-
Rodriguez PC, Popa X, Martinez O, Mendoza S, Santiesteban E, Crespo T, Amador RM, Fleytas R, Acosta SC, Otero Y, et al. A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung Cancer patients. Clin Cancer Res. 2016;22(15):3782–90.
-
Crombet Ramos T, Rodriguez PC, Neninger Vinageras E, Garcia Verdecia B, Lage Davila A. CIMAvax EGF (EGF-P64K) vaccine for the treatment of non-small-cell lung cancer. Expert Rev Vaccines. 2015;14(10):1303–11.
-
Neninger Vinageras E, de la Torre A, Osorio Rodriguez M, Catala Ferrer M, Bravo I, Mendoza del Pino M, Abreu Abreu D, Acosta Brooks S, Rives R, del Castillo Carrillo C, et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26(9):1452–8.
-
Garcia B, Neninger E, de la Torre A, Leonard I, Martinez R, Viada C, Gonzalez G, Mazorra Z, Lage A, Crombet T. Effective inhibition of the epidermal growth factor/epidermal growth factor receptor binding by anti-epidermal growth factor antibodies is related to better survival in advanced non-small-cell lung cancer patients treated with the epidermal growth factor cancer vaccine. Clin Cancer Res. 2008;14(3):840–6.
-
Gonzalez G, Crombet T, Torres F, Catala M, Alfonso L, Osorio M, Neninger E, Garcia B, Mulet A, Perez R, et al. Epidermal growth factor-based cancer vaccine for non-small-cell lung cancer therapy. Ann Oncol. 2003;14(3):461–6.
-
Sanchez L, Muchene L, Lorenzo-Luaces P, Viada C, Rodriguez PC, Alfonso S, Crombet T, Neninger E, Shkedy Z, Lage A. Differential effects of two therapeutic cancer vaccines on short- and long-term survival populations among patients with advanced lung cancer. Semin Oncol. 2018;45(1–2):52–7.
-
Saavedra D, Garcia B, Lorenzo-Luaces P, Gonzalez A, Popa X, Fuentes KP, Mazorra Z, Crombet T, Neninger E, Lage A. Biomarkers related to immunosenescence: relationships with therapy and survival in lung cancer patients. Cancer Immunol Immun. 2016;65(1):37–45.
-
Alonso A, Van der Elst W, Molenberghs G. Validating predictors of therapeutic success: a causal inference approach. Stat Model. 2015;15(6):619–36.
-
Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25(1):127–41.
-
Bilen MA, Martini DJ, Liu Y, Lewis C, Collins HH, Shabto JM, Akce M, Kissick HT, Carthon BC, Shaib WL, et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer. 2019;125(1):127–34.
Acknowledgements
We thank all participating patients and their families, as well as staffs of all the institutions involved in this study.
Funding
This study is part of the research activities of the Cuban-Flemish Training and Research Program in Data Science and Big Data Analysis, supported by Flemish Interuniversity Council (VLIR).
Author information
Affiliations
Contributions
PL, LS and AL conceived the study, WVE, AA and GM developed the methodology. PL, LS WVE, AA and GM Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis). AL, DS and TC participate in the clinical interpretation of the data. All authors critically revised subsequent drafts of the manuscript and approved the final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The trial protocol, informed consent, investigator brochure, and case report forms were approved by the ethic boards from each participating institution, including the main investigation site: Hermanos Ameijeiras Hospital and by the Cuban Regulatory Agency (CECMED). Informed consent was obtained from each subject before entering in the study. The trial was conducted in accordance with the principles of the declaration of Helsinki and Good Clinical Practice guidelines. It was registered at the Cuban Registry of Clinical Trials, a WHO-validated public registry http://www.who.int/ictrp/network/rpcec/en, trial number RPCEC00000161).
Consent for publication
This manuscript does not contain any details, images, or videos that might leed to identification of an individual patient.
Competing interests
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gerelateerde artikelen
- Longkanker: CIMAvax-EGF vaccin geeft uitstekende resultaten op overall overleving en ziektevrije tijd bij longkanker en andere vormen van kanker met EGFR mutatie
- Longkanker: Specifieke bloedwaarden en biomarkers die wijzen op veroudering van immuunsysteem plus basale EGF-concentratie in bloed resulteerden in goede voorspellers van het succes van het CIMAvax-EGF vaccin bij gevorderde longkanker
- Longkanker: Peptide vaccin - L-BLP25 - tegen uitgezaaide inoperabele niet-klein-cellige longkanker - fase IIIB - effectief. Nieuwe fase III studie moet resultaten bevestigen
- Longkanker: Succes van vaccin tegen longkanker (ook in Leuven /Brussel) wordt alleen bereikt met hulp van Mage=A3 eiwit tegen terugkeer van operatief verwijderde niet-klein-cellige longkanker. Artikelupdate 14 juni 2008
- Longkanker: Immuuntherapie met Nivolumab + chemotherapie geeft op 3-jaars meting bij patienten met operabele niet-kleincellige longkanker (NSCLC) betere ziektevrije overleving (57 procent vs 43 procent) in vergelijking met alleen chemotherapie




Plaats een reactie ...
Reageer op "Longkanker: Specifieke bloedwaarden en biomarkers die wijzen op veroudering van immuunsysteem plus basale EGF-concentratie in bloed resulteerden in goede voorspellers van het succes van het CIMAvax-EGF vaccin bij gevorderde longkanker"