18 juni 2020: Zie ook dit artikel:
12 maart 2018: Bron: Evid Based Complement Alternat Med. 2017; 2017: 3970601. Published online 2017 Aug 6.
Compound Danxiong Granules (CDG), een zalf samengesteld uit poeder van verpulverde verschillende Chinese kruiden (Rhizoma Chuanxiong, Paeonia suffruticosa Andr., Cortex Phellodendri, Geranium sibiricum L., and Flos Carthami) twee keer daags gebruikt tijdens chemotherapie of behandeling met gerichte medicijnen (targeted therapie) voorkomt sterk bepaalde bijwerkingen van de huid en dan met name springt het hand- voetsyndroom eruit, maar ook acne en infecties van nagelranden en tenen en vingers.
Bijwerkingen van de huid, ook wel dermatologische toxiciteiten genoemd, zijn vaak de oorzaak dat de dosering moet worden aangepast van de medicijnen (chemo) die worden gegeven of soms zelfs leiden die ertoe dat de behandeling moet worden stopgezet. Ik denk bv. dat veel mensen wel weten wat het hand- en voet syndroom (pijnlijke rood "verbrande" voeten en handen) voor pijn kan veroorzaken. Een beschadiging die eenmaal veroorzaakt nooit meer echt volledig weggaat.
Uit het studierapport een voorbeeld van drie patiënten voor en na de zalf te hebben gebruikt.
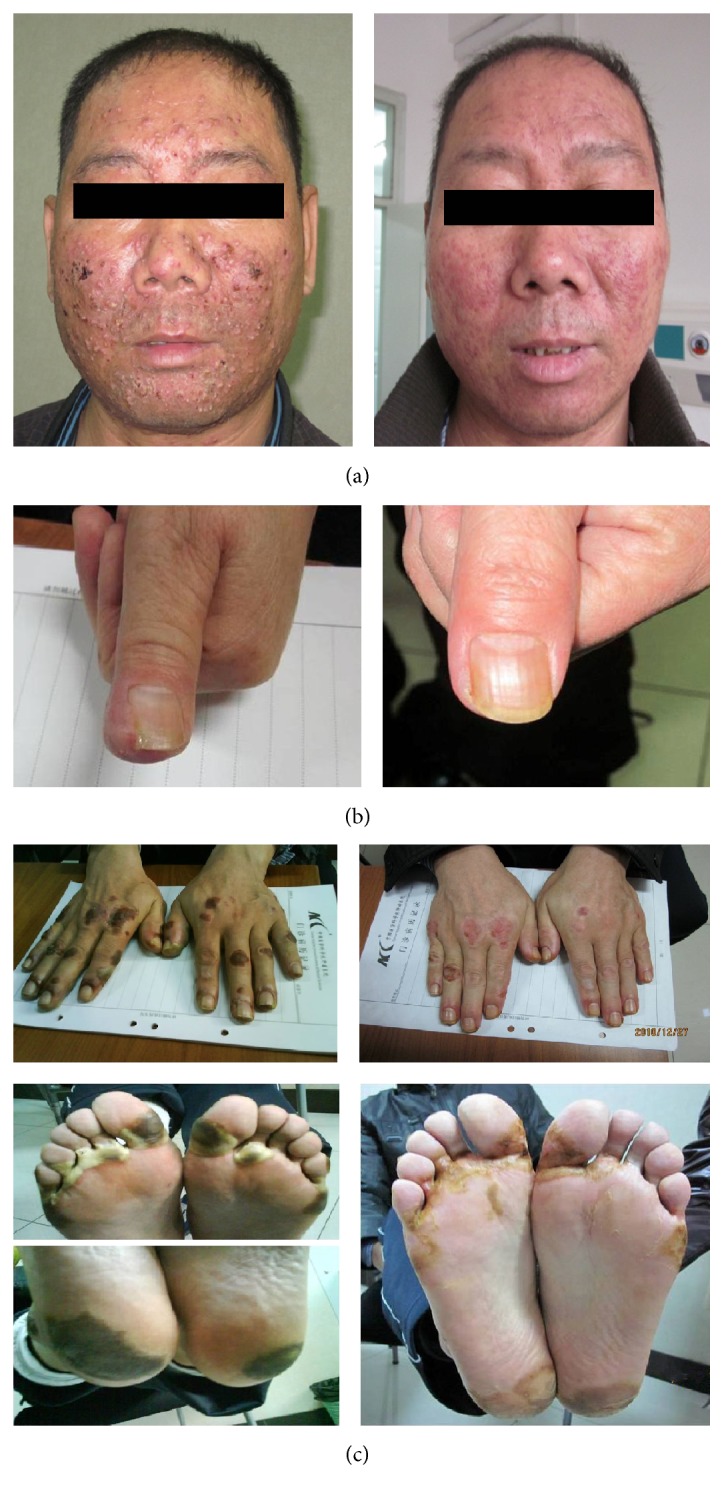
Three representative patients before and after treatment. (a) Acneiform eruption associated with Erlotinib in a patient with lung cancer. Before treatment (left), the face displayed swelling, itching, pain, purulent heads, and hemorrhage (Grade III). After 10 days of topical washes with CDG (right), the rash nearly healed. (b) Paronychia associated with Erlotinib in a patient with lung cancer. Before treatment (left), the nail exhibited pain, redness, and swelling (Grade III). After 10 days of topical washes with CDG (right), the tissues surrounding the nail recovered. (c) Hand-foot skin reaction associated with Sorafenib in a patient with hepatocellular carcinoma. Before treatment (left), skin areas on both hand and foot displayed redness, swelling, foaming, bleeding blister, and pain (Grade III). After 10 days of topical washes with CDG (right), the lesions were nearly healed.
In een gerandomiseerde, dubbelblinde, placebo-gecontroleerde studie is onderzocht wat het effect van een zalf Compound Danxiong Granules (CDG) zou zijn voor die zogeheten dermatologische toxiciteiten veroorzaakt door de behandelingen. En het bleek een groot succes. Het hand- en voet syndroom trad niet op bij 77 procent (52 uit 67 patienten) die deze zalf hadden gebruikt tegenover 27 procent bij de placebogroep (9 uit 33 patiënten). Ook kwam er in de CDG groep minder acne voor (69,23 vs 30,78 procent) en minder paronychia (infectie van de huid bij nagels en aan vingers en tenen) (68,42 vs 22,22 procent).
Table 3
Comparison of clinical effective rate between treatment groups.
| Group | Total effective rate | Treatment failure rate | Chi-square | P value |
|---|---|---|---|---|
| Compound Danxiong Granules (n = 67) | 52 (77.61%) | 15 (22.39%) | 23.55 | <0.0001 |
| Placebo (n = 33) | 9 (27.27%) | 24 (72.73%) |
The total effective rate was 77.61% (52/67) in the CDG group and 27.27% (9/33) in the placebo group (P < 0.0001). Compared to the placebo treatment, CDG treatment achieved a higher total effective rate for hand-foot skin reaction (95.45% versus 27.27%), acneiform eruption (69.23% versus 30.78%), and paronychia (68.42% versus 22.22%).
Conclusie: De onderzoekers concluderen dan ook terecht dat de CDG-zalf (Compound Danxiong Granules ) ernstige bijwerkingen van de huid als gevolg van chemotherapie of targeted therapie kan voorkomen. (Our study suggests that topical application of CDG can effectively attenuate dermatologic toxicities associated with targeted anticancer therapy. The treatment effect of CDG was more pronounced in hand-foot skin reaction.)
Ik kan maar 1 foto vinden van CDG en weet neit of de zalf ook hier te koop is:
www.jojbuy.com maar wellicht weet de natuurapotheek wel hoe aan die zalf te komen.

Het volledige studierapport: Efficacy of Topical Compound Danxiong Granules for Treatment of Dermatologic Toxicities Induced by Targeted Anticancer Therapy: A Randomized, Double-Blind, Placebo-Controlled Trial is gratis in te zien.
Het abstract staat hieronder met referentielijst.
Topical application of Compound Danxiong Granules (CDG) can effectively attenuate dermatologic toxicities associated with targeted anticancer therapy. The treatment effect of Compound Danxiong Granules (CDG) was more pronounced in hand-foot skin reaction.
Efficacy of Topical Compound Danxiong Granules for Treatment of Dermatologic Toxicities Induced by Targeted Anticancer Therapy: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Dermatologic toxicities resulting in dose reduction or discontinuation of treatment pose challenges for targeted anticancer therapies. We conducted this randomized, double-blind, placebo-controlled trial to investigate the efficacy of topical application of Compound Danxiong Granules (CDG) for treatment of dermatologic toxicities associated with targeted anticancer therapies. One hundred and ten patients with dermatologic toxicities induced by targeted anticancer therapies were randomly assigned to CDG or placebo group. Each crude herb (Rhizoma Chuanxiong, Paeonia suffruticosa Andr., Cortex Phellodendri, Geranium sibiricum L., and Flos Carthami) was prepared as an instant herbal powder. Application of the CDG via topical washes lasted 20 minutes, twice daily, for 10 days. The primary outcome was the total effective rate, defined as reduction in at least one grade of skin toxicity. The total effective rate was 77.61% (52/67) in the CDG group and 27.27% (9/33) in the placebo group (P < 0.0001). Compared to the placebo treatment, CDG treatment achieved a higher total effective rate for hand-foot skin reaction (95.45% versus 27.27%), acneiform eruption (69.23% versus 30.78%), and paronychia (68.42% versus 22.22%). Topical application of CDG can effectively attenuate dermatologic toxicities induced by targeted anticancer therapies. The effect of CDG was more pronounced in hand-foot skin reaction.
References
Plaats een reactie ...
1 Reactie op "Danxiong Granules zalf (CDG), een mix van Chinese kruiden voorkomt voor 75 procent hand- en voetsyndroom veroorzaakt door chemotherapie"
Gerelateerde artikelen
- Adressen van betrouwbare TCM artsen en acupuncturisten
- Algemeen:TCM - Traditionele Chinese Kruiden aanvullend bij een langdurige radiotherapeutische behandeling van buiktumoren geeft hoog significant een betere kwaliteit van leven en een significant betere 3 jaars overleving (67,55%) dan bij bestraling alleen
- TCM - Traditionele Chinese Medicijnen - kruiden verminderen misselijkheid en braken naast tropisetron veroorzaakt door chemotherapie bij patienten met niet-kleincellige longkanker en verbeteren kwaliteit van leven
- TCM = traditionele Chinese Medicijnen in combinatie met een Hipec = hyperthermic intraperitoneal chemotherapy heeft gunstig effect op met name vocht in de buik (maligne ascites)
- Artritis - reuma: Chinese kruid, thunder god vine van de lei gong teng plant geeft significant betere pijnverlichting bij reumatische artritis dan het standaard middel methotrexate
- Astralagus: Traditionele Chinese medicijnen (TCM) met astragalus injectie aanvullend op westerse medicijnen bij patienten met buikvlieskanker geeft 20 procent betere 3-jaars overleving in vergelijking met alleen westerse medicijnen copy 1
- Chinese kruidenmix - Fufang Zaofan - vermindert in gerandomiseerd onderzoek leucopenie als bijwerking van radiotherapie plus chemo
- Chinese plantenmix Taohongsiwu afkooksel om te weken geneest of vermindert sterk het door chemotherapie veroorzaakte hand-voet-syndroom en is veel beter dan oraal vitamine B6.
- Danxiong Granules zalf (CDG), een mix van Chinese kruiden voorkomt voor 75 procent hand- en voetsyndroom veroorzaakt door chemotherapie
- Kanglaite - Shenqui Fuzheng injecties, een mix van Chinese kruiden en plantenextracten. Een overzicht
- LCS101 een kruidenmix van 14 kruiden is ook effectief bij kankerpatienten met solide tumoren en naast bestraling - radiotherapie en verkrijgbaar in Nederland
- Ginseng in een behandeling van kanker en tegengaan van bijwerkingen. Een overzichtsartikel voor artsen en voedingsdeskundigen.
- Borstkanker: TCM - Traditionele Chinese medicijnen / kruiden gebruik naast chemo geeft 12 procent minder doden aan gevorderde uitgezaaide borstkanker over 10 jaar geregistreerd. copy 1
- Bottumoren: Het Chinese kruid Guliu als aanvulling in capsules naast inwendige bestraling (SR 89 therapie - strontiumisotoop) bij kankerpatiënten met botuitzaaiïngen en bottumoren geeft significant beter therapeutisch effect en veel minder bijwerkingen
- Darmkanker: Chinese kruiden - injecties met Shenqui (zit ook in Artemisinin) - als aanvulling op chemo bij vergevorderde darmkankerpatienten geeft een significant betere kwaliteit van leven en mediaan een significante verbetering van de overlevingstijd.
- Leverkanker: TCM, traditionele niet toxische Chinese medicijnen geven beter therapeutisch effect en significant veel betere kwaliteit van leven dan chemo bij patienten met gevorderde primaire leverkanker.
- Longkanker: TCM - traditionele Chinese medicijnen - Chinese kruiden als behandeling van longkanker. Mono of aanvullend bij chemo of bestraling. Een overzicht. Update 16 mei 2010 copy 1
- Maagkanker: Fuzheng Yiliu decoction - een Chinees kruid - als aanvulling verhoogt significant de effectiviteit van chemo bij maagkanker en vermindert significant de bijwerkingen, ook op het beenmerg en de spijsvertering.
- Mond- en keelkanker: TCM - Traditionele Chinese kruiden verminderen significant de bijwerkingen van bestraling van neus- en keelkanker.
- Slokdarmkanker: Shenyi (een van ginseng afgeleid Chinees kruid) gegeven naast gemzar en cisplatin vermindert bijwerkingen van chemo en verbetert overleving bij slokdarmkanker.
- Chinese medicijnen - TCM: traditionele Chinese medicijnen - Chinese kruiden therapeutisch of preventief bij alle vormen van kanker. Een overzicht van recente ontwikkelingen en belangrijke studies en artikelen



De zalf werkt erg goed bij haar dus echt een aanrader! Haar pijn is veel minder en de plekken ook.