Mocht u kanker-actueel de moeite waard vinden en ons willen ondersteunen om kanker-actueel online te houden dan kunt u ons machtigen voor een periodieke donatie via donaties: https://kanker-actueel.nl/NL/donaties.html of doneer al of niet anoniem op - rekeningnummer NL79 RABO 0372931138 t.n.v. Stichting Gezondheid Actueel in Amersfoort. Onze IBANcode is NL79 RABO 0372 9311 38
Elk bedrag is welkom. En we zijn een ANBI instelling dus uw donatie of gift is in principe aftrekbaar voor de belasting. En als donateur kunt u ook korting krijgen bij verschillende bedrijven:
19 september 2015: Bron: Science Translational Medicine
Immuuntherapie met gemanipuleerde T-cellen zorgde al eerder voor opmerkelijke resultaten, zoals in 2011 en 2013 bij een aantal patiënten met CLL - Chronische lymfatische leukemie. Nu blijkt dat van de patiënten die toendertijd goed reageerden met een gedeeltelijke of complete remissie deze remissies duurzaam zijn. Bij 8 van de 14 patiënten blijkt na 4,5 jaar nog steeds geen sprake van een recidief. En dan te bedenken dat alle patiënten zwaar voorbehandeld waren voordat ze in aanmerking kwamen voor deze vorm van immuuntherapie met gemanipuleerde T-cellen.
Zie verderop uitgebreidere beschrijving van deze aanpak en studie. Of lees dit studierapport eens dat mooi overzicht geeft waar CAR-T cells al zijn toegepast en ook hoe het werkt en de resultaten daarmee: Advantages and applications of CAR-expressing natural killer cells
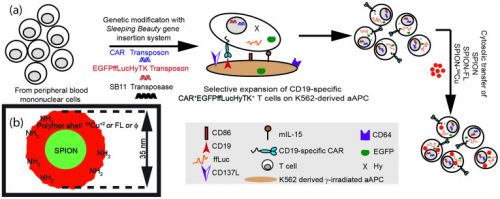
Foto: Werkingsmechanisme van gemanipuleerde T-cellen
Hier de laatste details uit deze studie van de Universiteit van Pennsylvania gepubliceerd door Porter et al in Science Translational Medicine, Nieuwste abstract staat onderaan artikel
Nieuwste gegevens:
De nieuwe publicatie geeft de nieuwste gegevens van de 14 patiënten met CLL - Chronische Lymfatische Leukemie die enkele jaren geleden positief reageerden op de immuuntherapie met gemanipuleerde T-cellen die startte in 2010.
De overall response was toen 57%. Alle patiënten die de immuuntherapie hadden gekregen hadden een recidief of vergevorderde ziekte ondanks meerdere standaard behandelingen zoals vastgelegd in de richtlijnen van de FDA. en enkele patiënten zouden in aanmerking komen voor een stamceltransplantatie.
- 4 patiënten (29%) in the studie bereikten een complete remissie. Een patiënt overleed terwijl deze in complete remissie verkeerde na 21 maanden aan een niet aan de behandeling gerelateerde oorzaak, maar aan een infectie veroorzaakt door de verwijdering van een huidkankertumor op zijn been. De andere drie patiënten bleven/zijn in leven tot het moment van deze analyse met geen enkel teken van een recidief van hun leukemie. Op moment van opmaken van de resultaten respectievelijk 28, 52 en 53 maanden na de immuuntherapie zonder verdere andere behandelingen.
- 4 andere patiënten (29%) realiseerden een gedeelteljike remissie van hun ziekte door de behandeling van mediaan 7 maanden zonder recidief of progressie van de ziekte. Twee van deze patiënten overleden tijdens de studie follow-up aan hun ziekte respectievelijk na 10 maanden en 27 maanden na de immuuntherapie had plaatsgevonden.
- 1 patiënt overleed aan de gevolgen van een longembolie 6 maanden na de immuuntherapie.
- Bij 1 patiënt gaf de ziekte progressie te zien na 13 maanden maar deze patiënt bleef nog 36 maanden in leven door andere behandelingen.
- 6 patiënten (43%) reageerden niet op de immuuntherapie en zagen progressie van hun ziekte tussen 1 en 9 maanden na de immuunbehandeling. testen wezen uit dat deze patiënten minder geod reageerden op de T-cel injecties dan de patiënten die wel goed reageerden. Twee van deze patiënten overleden later aan de gevolgen van hun ziekte. 4 patiënten kregen andere behandelingen.
Voor abstract van deze laatste studie zie onderaan artikel. Het volledige studierapport: Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia is tegen betaling in te zien.
11 december 2013: Bron: Congres American Society of Hematology ( ASH ) 2013
Immuuntherapie met gemanipuleerde T-cellen zorgt voor spectaculaire resultaten bij patiënten met vergevorderde vormen van leukemie - ALL, CLL, en lymfomen.
Bij 15 patiënten met zogeheten B-cell lymfomen kwamen 7 patiënten alsnog in een complete remissie en 5 in een gedeeltelijke remissie (minimaal 50 % vermindering van tumoromvang)
Bij kinderen met ALL - Acute lymfatische leukemie werden 22 patiënten behandeld en daarvan kwamen er 19 in een complete remissie waarvan er 5 alsnog later een recidief kregen, maar 14 bereikten dus een langdurige complete remissie (klinisch kankervrij). Zie ook deze studie: Immuuntherapeutische aanpak met receptor-gemodificeerde T-cellen leidt tot totale remissies bij agressieve acute lymfatische leukemie (ALL) bij zowel kinderen als volwassenen
En ook bij patiënten met CLL - chronische lymfatische leukemie werden 32 patiënten behandeld waarvan er 15 patiënten een gedeeltelijke remissie bereikten en 7 patiënten een complete remissie. En dan te bedenken dat alle deelnemende patiënten voor deze behandeling al zijn behandeld met verschillende andere behandelingen als chemo, stamceltransplantatie enz.
Deze nieuwe vorm van behandelen houdt in dat T-cellen worden gehaald uit de lymfklieren en bloed van de patiënten. Daarna worden in een laboratorium deze T-cellen onderworpen aan een programma waarin deze T-cellen in contact worden gebracht met een CD 19 antigen, dat veel voorkomt op de oppervlakte van leukemie cellen. Dit proces wordt chimere antigeen receptor ( CAR ) genoemd. Daarna worden deze bewerkte T-cellen teruggebracht in de patiënt.
In het lichaam, als de aanpak aanslaat, veroorzaakt dit CD 19 antigen een zogeheten virale vector mechanisme in de cellen die - nadat de cellen zijn vergrendeld op de leukemiecellen zelf - deze T-cellen activeert en doet groeien en zich vermenigvuldigen, zodat ze alle resterende leukemie cellen opsporen en vernietigen. In feite werkt dendritische celtherapie min of meer ook zo als er tumorweefsel aanwezig is om te kunnen "logen" zoals dat heet.
De resultaten zijn dus echt spectaculair want bij veel patiënten waar de T-cellen waren ingebracht en die goed reageeerden op deze aanpak verdwenen de kankercellen al na 1 behandeling, aldus James Kochenderfer, MD, from the Experimental Transplantation and Immunology Branch of the National Cancer Institute (NCI) in Bethesda, Maryland.
Wel kan deze behandeling leiden tot ernstige bijwerkingen, die soms vereist dat de patiënt verblijft in een intensive care unit. Echter de bijwerkingen zijn tijdelijk en meestal na 2 dagen onder controle of verdwijnen vanzelf. Zodra de T-cellen beginnen uit te breiden in het lichaam, ontwikkelen bijna alle patiënten een vertraagde cytokine afgifte en macrofaag activering, wat kan leiden tot acute toxiciteit, hoge koorts , verhoogde bloeddruk, ademhalingsmoeilijkheden, delirium, afasie en neurologische toxiciteit. Echter de patiënten herstellen snel , meestal binnen 2 dagen , en de symptomen zijn binnen ongeveer 3 weken opgelost , aldus Dr Kochenderfer.
Op het Congres American Society of Hematology ( ASH ) 2013 werd al gespeculeerd dat deze aanpak al in 2016 voor patiënten beschikbaar komt, al waren er ook anderen die pas 2020 als datum van invoering zagen. Zoals met veel op receptoren en mutaties gerichte benaderingen zal het afhangen van de toezichthoudende instanties FDA en EMU bv. of die bereid zijn de eisen voor evidence based medicin aan te passen.
Ik heb achtereenvolgens de originele abstracten van de verschillende studies hieronder geplaatst:
Chimeric Antigen Receptor Modified T Cells Directed Against CD19 (CTL019 cells) Have Long-Term Persistence and Induce Durable Responses In Relapsed, Refractory CLL
Source: 55th. ASH Meeting and Exhibition
Session: 642. CLL: Therapy, excluding Transplantation: Poster III
Chimeric antigen receptors (CARs) combine the antigen recognition domain of an antibody with intracellular signaling domains into a single chimeric protein. CD19 is an ideal target for CARs since expression is restricted to normal and malignant B cells. Inclusion of the CD137 (4-1BB) signaling domain results in potent antitumor activity and in-vivo persistence of anti-CD19 CAR-modified T cells in mice. Lentiviral transduction into T cells facilitates strong surface expression of the CAR. We reported anti-tumor activity of CAR-modified autologous T cells targeted to CD19 (CTL019 cells) in 3 patients (pts) with CLL with relatively short follow up (Porter, et al NEJM 2011; Kalos et al Sci Trans Med 2011). We now report on outcomes and longer follow up from our pilot study treating 14 pts with relapsed, refractory CLL.
METHODS
Autologous T cells collected by leukapheresis were transduced with a lentivirus encoding anti-CD19 scFv linked to 4-1BB and CD3-ζ signaling domains. Gene-modified T cells were expanded and activated ex-vivo by exposure to anti-CD3/CD28 beads. Pts had to have relapsed or persistent disease after at least 2 previous treatments (1 prior therapy for patients with p53 mutation) and progressed at least within 2 years of their last therapy. All pts received lymphodepleting chemotherapy ending 3-5 days before T cell infusion. The target dose of cells was 5 x 109 mononuclear cells with an expected transfection efficiency of 10-40% (total CTL019 dose 5x108 – 2 x 109 total cells). Cell infusions were planned over 3 days (10% on day 1, 30% of day 2, and 60% on day 3) but were held for fevers or other toxicity.
RESULTS
14 patients were treated on this pilot study including 12 men and 2 women with a median age of 67 (51-78). Pts had received a median of 4 prior therapies (1-10) and 6 pts had a mutation of p53. All pts had active disease at the time of CTL019 cell infusion. Lymphodepleting chemotherapy was Fludarabine/cyclophosphamide (3), pentostatin/cyclophosphamide (5), or bendamustine (6). A median of 7.5 x 108 total cells (range 1.7-50), corresponding to 1.4 x 108(range 0.14-5.9) genetically modified cells were infused over day 0, 1 and 2.
There were no infusional toxicities >grade 2 though 6 pts developed fevers within 24 hrs of infusion #1 (3) or #2 (3) and did not receive additional CTL019 cells. Median follow-up as of July 15, 2013 was 9.4 mo (4-35) for all pts and 16 mo (5-35) for the 8 responding pts. 3 patients (21%) achieved a CR (follow-up 11, 34, and 35 mo), 5 (36%) achieved a PR (med follow up 11 mo, range 5-27 mo) and 6 (43%) had no response, for an overall major response rate of 57%. 2 of 5 pts with a PR progressed 4 mo after infusion with CD19+ CLL, and no patient with a CR has relapsed.
Comparing responders to non-responders, there has been no association between response and patient age (66 vs 67 yrs), number of prior therapies (median 4 each), cell dose (7.5 vs 11.5 x 108MNC), or p53 mutation (3/8 vs 3/6, p>0.9), implying that within the dose ranges studied, there is no obvious dose:response relationship.
All responding pts developed a delayed cytokine release syndrome (CRS), concurrent with peak T cell expansion, and was manifested by fever, and variable degrees of nausea, anorexia, myalgias, and transient hypotension and hypoxia. Detailed cytokine analysis showed marked increases from baseline values of IL6, IFN-γ, and IL2R, while no significant elevation in systemic levels of TNFα or IL2 were observed. The CRS required intervention in 5 patients. Treatment was initiated for hemodynamic or respiratory instability and was rapidly reversed in all cases with corticosteroids in 1 pt and the IL6-receptor antagonist tocilizumab (4 pts); 3 of these 4 pts also received 1 or 2 doses of corticosteroids. Persistence of CTL019 cells has been detected by flow cytometry in all 6 pts with ongoing responses 5-35 months after infusion, and all patients have sustained B cell aplasia without any unusual infectious complications.
CONCLUSIONS:
CTL019 cells are autologous T cells genetically engineered to express an anti-CD19 scFv coupled to 4-1BB/CD3-ζ signaling domains. These cells can undergo robust in-vivo expansion and can persist for at least 3 yrs. CTL019 therapy is associated with a significant CRS that responds rapidly to anti-cytokine treatment. CTL019 cells can induce potent and sustained responses (8/14) for patients with advanced, relapsed and refractory CLL regardless of p53 mutation status.
Disclosures: Porter: Novartis: Patents & Royalties, Research Funding; Genentech: Spouse employment, Spouse employment Other. Off Label Use: CTL019 cells to treat CLL. Kalos: Adaptive biotechnologies: Member scientific advisory board , Member scientific advisory board Other; Novartis corporation: CART19 technology, CART19 technology Patents & Royalties. Grupp: Novatis: Research Funding. Lledo: Novartis: Research Funding. Chew: Novartis: Patents & Royalties. Zheng: Novartis: Patents & Royalties. Levine: Novartis: cell and gene therapy IP, cell and gene therapy IP Patents & Royalties. June: Novartis: Patents & Royalties, Research Funding.
Randomized, Phase II Dose Optimization Study Of Chimeric Antigen Receptor Modified T Cells Directed Against CD19 (CTL019) In Patients With Relapsed, Refractory CLL
Source: 55th. ASH Meeting and exposition 2013
Type: Oral
Session: 642. CLL: Therapy, excluding Transplantation: Novel Approaches to CLL Therapy
Patients (pts) with relapsed, and/or refractory (R/R) CLL have a poor prognosis with few effective treatment options. We have shown that infusion of autologous T cells genetically modified to express a chimeric antigen receptor (CAR) consisting of an external anti-CD19 domain, with the CD3ζ and 4-1BB signaling domains (CTL019 cells), can mediate potent anti-tumor effects in pts with advanced, relapsed refractory CLL. In our initial pilot study, doses of 1.7-50, x 108 mononuclear cells, corresponding to 0.14-5.9 x 108genetically modified cells, were given as a split dose infusion on days 0, 1 and 2 to 14 pts with R/R CLL and overall response rate (PR plus CR) was 57%. The majority of responses were sustained, and associated with marked expansion and long-term persistence of transduced cells. Notably, there was no obvious dose:reponse or dose:toxicity effect noted over a wide range of cell doses. To better define an optimal CTL019 cell dose, we are performing a randomized phase II study of 2 doses of CTL019 cells in pts with R/R CLL.
Methods:
Pts with R/R CLL are randomly assigned to receive either 5x108 vs. 5x107transduced CTL019 cells, with the rationale that both doses induced CRs in pts on our initial pilot trial. In the initial stage, 12 evaluable pts will be treated in each arm and in stage 2, an additional 8 pts will be treated with the selected dose level. Pts have to have relapsed or persistent disease after at least 2 previous treatments and progress within 2 years of their last therapy. All pts receive lymphodepleting chemotherapy ending 3-5 days before T cell infusion. Cell infusions are given as a single dose.
Results:
As of 7/15/2013, 27 pts have been enrolled; T cells did not adequately expand in 3, 1 patient was not eligible after screening, and 10 pts have been treated including 7 men and 3 women with a median age of 63 yrs (range 59-76). 5 pts had a mutation of p53. All pts had active disease at the time of CTL019 cell infusion. Lymphodepleting chemotherapy was Fludarabine/cyclophosphamide (8), pentostatin/cyclophosphamide (1), or bendamustine (1). 4 pts have been randomized to the higher dose level (5 x 108 CTL019 cells) and 6 pts have been randomized to the lower dose level (5 x 107CTL019 cells). There were no significant infusional toxicities. Median follow-up as of July 15, 2013 was 3 mo (1.3-5) for all pts and 3.3 mo (1.3-4) for responding pts. 2 pts have achieved a CR and 2 pts achieved PR, both with clearance of CLL from the blood and marrow and >50 reduction in adenopathy, for an overall response rate of 40%. In other recipients of CTL019 cells, we have observed ongoing improvement in adenopathy over time implying there can be a continued anti-tumor response. No responding patient has progressed. Seven of 10 pts experienced a delayed cytokine release syndrome (CRS) manifested by symptoms that included high fevers, nausea, myalgias and in some cases, capillary leak, hypoxia, and hypotension, typically correlated with peak CTL019 cell expansion.
We have noted that the CRS accompanying CTL019 therapy has been associated with marked increases of serum IL6 and can be rapidly reversed with the IL6-receptor antagonist tocilizumab. The CRS required intervention in 2 pts, one who responded and one who did not respond to CTL019. Treatment was initiated for hemodynamic or respiratory instability and was effective in reversing signs and symptoms of CRS in both pts.
A preliminary analysis through July 15, 2013 does not yet suggest a dose:response or dose:toxicity relationship. 2 of 4 recipients of the higher dose CTL019 responded, and 2 of 6 recipients at the lower dose level responded. The 7 pts who experienced a CRS included all 4 responding pts and 3 pts who did not respond. The CRS occurred in 3/4 recipients of higher dose CTL019 cells and 4/6 of recipients of lower dose CTL019 cells. CTL019 expansion in-vivo and persistence over the follow up period was noted in all responding pts.
Conclusions:
In this ongoing dose optimization study of CTL019 cells, 4 of the first 10 pts treated have responded within 3 months. With short follow-up, as yet there is no suggestion that there is a dose:response or dose:toxicity relationship at the dose ranges being studied. These cells can undergo robust in-vivo expansion and from other studies (ASH 2013) can persist for at least 3 yrs. This trial confirms that CTL019 cells can induce potent responses for pts with advanced, relapsed and refractory CLL.
Disclosures: Porter: Novatis: IP and potential royalties with COI managed according to policies of the University of Pennsylvania, IP and potential royalties with COI managed according to policies of the University of Pennsylvania Patents & Royalties, Research Funding; Genentech: Spouse employment, Spouse employment Other. Off Label Use: CTL019 cells to treat CLL. Kalos: Novartis corporation: CART19 technology, CART19 technology Patents & Royalties; Adaptive biotechnologies: Member scientific advisory board , Member scientific advisory board Other. Grupp: Novartis: Research Funding. Chew: Novartis: Patents & Royalties. Shen: Novartis Pharmaceuticals: Employment, Equity Ownership. Wood: Novartis Pharmaceuticals: Employment, Equity Ownership. Litchman: Novartis Pharmaceuticals Corporation: Employment, Equity Ownership. Zheng: Novartis: Patents & Royalties. Levine: Novartis: cell and gene therapy IP, cell and gene therapy IP Patents & Royalties. June: Novartis: Patents & Royalties, Research Funding.
T Cells Engineered With a Chimeric Antigen Receptor (CAR) Targeting CD19 (CTL019) Produce Significant In Vivo Proliferation, Complete Responses and Long-Term Persistence Without Gvhd In Children and Adults With Relapsed, Refractory ALL
Source: 55th. ASH meeting and exposition
Type: Oral
Session: 614. Acute Lymphoblastic Leukemia: Therapy, excluding Transplantation: Novel Immune-Based Therapies and Novel Targets
CARs combine a single chain variable fragment (scFv) of an antibody with intracellular signaling domains into a single chimeric protein. We previously reported on CTL019 cells expressing a CAR with intracellular activation plus costimulatory domains. Infusion of these cells results in 100 to 100,000x in vivo proliferation, durable anti-tumor activity, and prolonged persistence in pts with B cell tumors, including 1 sustained CR in a patient with ALL (Grupp, et al. NEJM 2013). We now report on outcomes and longer follow up from our pilot studies treating 20 pts (16 children and 4 adults) with relapsed, refractory ALL.
METHODS
T cells were lentivirally transduced with a CAR composed of anti-CD19 scFv/4-1BB/CD3ζ, activated/expanded ex-vivo with anti-CD3/anti-CD28 beads, and then infused into pts with relapsed or refractory CD19+ ALL. 17/20 pts received lymphodepleting chemotherapy the week prior to CTL019 infusion. The targeted T cell dose range was 107 to 108 cells/kg with a transduction efficiency (TE) of 11-45%. On the adult protocol, the target dose was 5 x 109 total cells split over 3 days with a TE of 6-31%. 11 pts had relapsed ALL after a prior allogeneic SCT. T cells were collected from the pt, regardless of prior SCT status, and not from allo donors. All pts s/p allo SCT had to be 6 mos s/p SCT with no GVHD or GVHD treatment.
RESULTS
16 children median age 9.5 y (5-22y) and 4 adults median age 50y (26-60y) with CD19+ ALL were treated. One child had T cell ALL aberrantly expressing CD19. 14/16 pediatric pts had active disease or +MRD after chemotherapy on the day prior to CTL019 cell infusion, while 2 were MRD(-). 3 of 4 adults had active disease prior to lymphodepleting chemotherapy, while 1 was in morphologic CR. Lymphodepleting chemotherapy varied with most receiving a Cytoxan-containing regimen the week prior to CTL019. A median of 3.7x106 CTL019 cells/kg (0.7-18x106/kg) were infused over 1-3 days.
There were no infusional toxicities >grade 2, although 5 pts developed fevers within 24 hrs of infusion and did not receive planned subsequent infusions of CTL019 cells. 14 patients (82%) achieved a CR, including the patient with CD19+ T ALL, 3 did not respond, and 3 are pending evaluation. 11/17 evaluable pts have ongoing BM CR with median follow up 2.6 mo (1.2-15 mo). Three patients with a CR at 1 month have subsequently relapsed, 1 with CD19(-) disease. Median follow-up as of August 1, 2013 was 2.6 mo (1-15 mo) for all pts.
All responding pts developed some degree of delayed cytokine release syndrome (CRS), concurrent with peak T cell expansion, manifested by fever, with variable degrees of myalgias, nausea, anorexia. Some experienced transient hypotension and hypoxia. Detailed cytokine analysis showed marked increases from baseline values of IL6 and IFNγ (both up to 1000x), and IL2R, with mild or no significant elevation in systemic levels of TNFα or IL2. Treatment for CRS was required for hemodynamic or respiratory instability in 7/20 patients and was rapidly reversed in all cases with the IL6-receptor antagonist tocilizumab (7 pts), together with corticosteroids in 4 pts. Although T cells collected from the 11 pts who had relapsed after allo SCT were generally 100% of donor origin, no GVHD has been seen. Persistence of CTL019 cells detected by flow cytometry and/or QPCR in pts with ongoing responses continued for 1-15 months after infusion, resulting in complete B cell aplasia during the period of CTL019 persistence. Pts have been treated with IVIg without any unusual infectious complications. One child who entered a CR subsequently developed MDS with a new trisomy 8 in ALL remission and has gone to SCT, and 1 child developed a single leukemia cutis lesion at 6 mo, still BM MRD(-).
CONCLUSIONS:
CTL019 cells are T cells genetically engineered to express an anti-CD19 scFv coupled to CD3ζ signaling and 4-1BB costimulatory domains. These cells can undergo robust in-vivo expansion and can persist for 15 mo or longer in pts with relapsed ALL. CTL019 therapy is associated with a significant CRS that responds rapidly to IL-6-targeted anti-cytokine treatment. This approach has promise as a salvage therapy for patients who relapse after allo-SCT, and collection of tolerized cells from the recipient appears to have a low risk of GVHD. CTL019 cells can induce potent and durable responses for patients with relapsed/refractory ALL. Multicenter trials are being developed to test this therapy for ALL in the phase 2 setting.
Disclosures: Grupp: Novartis: Research Funding. Chew: Novartis: Patents & Royalties. Levine: Novartis: cell and gene therapy IP, cell and gene therapy IP Patents & Royalties. Litchman: Novartis Phamaceuticals: Employment, Equity Ownership. Rheingold: Novartis: Research Funding. Shen: Novartis Pharmaceuticals: Employment, Equity Ownership. Wood: Novartis Pharmaceuticals: Employment, Equity Ownership. June: Novartis: Patents & Royalties, Research Funding.
Long-Term Functional Persistence, B Cell Aplasia and Anti-Leukemia Efficacy In Refractory B Cell Malignancies Following T Cell Immunotherapy Using CAR-Redirected T Cells Targeting CD19
Source: 55th. ASH meeting and exposition
Type: Oral
Session: 801. Gene Therapy and Transfer: Progress in vector development and gene therapy of acquired diseases
Recent advances in T cell engineering have enabled clinical trials to evaluate the potential for adoptive transfer of T cells to target malignancy. A single treatment with engineered gene-modified T cells has the potential to generate potent and long-lasting anti-tumor immunity. We have reported initial clinical data on the use of T cells engineered to express a Chimeric Antigen Receptor (CAR) that targets CD19 (CTL019) in patients with advanced treatment-refractory CLL and pediatric ALL (Porter, et al NEJM 2011; Kalos et al. Sci Trans Med 2011, Grupp et al. NEJM 2013). The initial cohort of patients is now disease free between 1 and 3 years post-infusion. In this report we present data on the functional persistence, trafficking, and bioactivity of CTL019 cells from the initial cohort of CLL patients, a larger and more recently treated cohort of CLL patients, a cohort of pediatric ALL patients, as well as an initial cohort of adult ALL patients.
METHODS
CAR engineering of patient T cells was accomplished by lentivirus transduction followed by in-vitro expansion using anti-CD3 and anti-CD28 antibody-coated beads. Persistence of gene-modified T cells was assessed by quantitative PCR. CAR19 surface expression was detected by flow. B cell aplasia was evaluated by multiparametric flow cytometry. Multiplex cytokine analyses were performed using LuminexTMtechnology.
RESULTS
Detailed clinical outcomes for each patient cohort will be reported separately at this meeting (Porter, D.L.et al, Grupp S. et al). In summary, as of July 15, 2013: For CLL: A total of 24 adult patients with advanced relapsed and/or treatment-refractory CLL have been treated under two separate protocols. To date 5 patients have achieved ongoing CR, 7 patients PR and 12 patients were NR at the primary endpoint (within 3 months post infusion). For ALL (pediatric): A total of 14 pediatric patients with treatment refractory ALL have been treated and were evaluable under a single protocol. To date 8 patients have ongoing CR, 4 patients have relapsed including 2 with CD19-negative disease and 2 patients were NR. For ALL (Adult): A total of 3 adult patients with advanced relapsed and/or treatment-refractory ALL have been treated under a single protocol. All 3 patients have ongoing CR; one patient went into an allogeneic transplant while in CTL019-induced CR.
In all patients with CR, robust in vivo expansion of CTL019 cells was observed, as assessed by both molecular and flow cytometric analysis, followed by contraction and in all but one patient ongoing stable persistence of engineered cells, elimination of tumor B cells and ongoing B cell aplasia in blood and marrow at all evaluated time points (minimum 3 months, maximum ongoing at 35 months). In CR patients, peak marking exceeded 5% of total CD3+. Patients with PR demonstrated less robust in-vivo expansion accompanied by transient B cell elimination, while NR patients demonstrated minimal in-vivo expansion.
Clinical MRD analysis in patients was supplemented with Illumina-HiSeq/MiSeq-deep sequencing based molecular MRD analysis. These analyses demonstrate that CRs were accompanied with deep and sustained molecular remissions as assessed by the absence of the tumor specific clonotype defined in the enrollment samples.
All patients in CR experienced on-target cytokine release syndrome (CRS) and macrophage activation syndrome (MAS). Multiplex-cytokine analysis of serum samples from patients in CR demonstrated a broad pro-inflammatory signature with significant elevation in a subset of soluble immune modulators including IFN-g, IL-6, and IL2ra. In contrast, patients with NR did not have elevated serum cytokines. Elevation of cytokines coincided with expansion of CTL019 cells, elimination of B cells, and toxicity suggesting the potential for a cytokine-based diagnostic signature to monitor CTL019 treatment efficacy.
CONCLUSIONS
Adoptive transfer of CTL019 cells engineered to express CD137 and TCR-zeta signaling domains can result in in-vivo expansion, homing to marrow, and long-term functional persistence of engineered cells, accompanied by ongoing complete clinical responses and long-term B cell aplasia in a substantial fraction of patients with advanced, refractory and high risk CLL and relapsed refractory pre- B cell ALL. These results highlight the potential of CTL019 therapy to effectively target CD19-positive malignancy.
Disclosures: Kalos: Novartis corporation: CART19 technology, CART19 technology Patents & Royalties; Adaptive biotechnologies: Member scientific advisory board , Member scientific advisory board Other. Zheng: Novartis: Patents & Royalties. Levine: Novartis: cell and gene therapy IP, cell and gene therapy IP Patents & Royalties. Grupp: Novartis: Research Funding. June: Novartis: Patents & Royalties, Research Funding.
Long-Term Remissions Reported in CLL Personalized Cell Therapy Trial with CAR T-cells
Gerelateerde artikelen



Plaats een reactie ...
Reageer op "Leukemie: Immuuntherapie met gemanipuleerde T-cellen geeft spectaculair goede resultaten bij patienten met vergevorderde leukemie en B-cel lymfomen copy 2"